Nesprins are mechanotransducers that discriminate epithelial-mesenchymal transition programs
- PMID: 32790861
- PMCID: PMC7659719
- DOI: 10.1083/jcb.201908036
Nesprins are mechanotransducers that discriminate epithelial-mesenchymal transition programs
Abstract
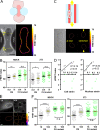
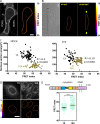
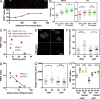
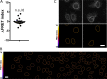
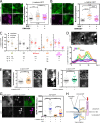
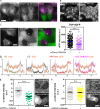
LINC complexes are transmembrane protein assemblies that physically connect the nucleoskeleton and cytoskeleton through the nuclear envelope. Dysfunctions of LINC complexes are associated with pathologies such as cancer and muscular disorders. The mechanical roles of LINC complexes are poorly understood. To address this, we used genetically encoded FRET biosensors of molecular tension in a nesprin protein of the LINC complex of fibroblastic and epithelial cells in culture. We exposed cells to mechanical, genetic, and pharmacological perturbations, mimicking a range of physiological and pathological situations. We show that nesprin experiences tension generated by the cytoskeleton and acts as a mechanical sensor of cell packing. Moreover, nesprin discriminates between inductions of partial and complete epithelial-mesenchymal transitions. We identify the implicated mechanisms, which involve α-catenin capture at the nuclear envelope by nesprin upon its relaxation, thereby regulating β-catenin transcription. Our data thus implicate LINC complex proteins as mechanotransducers that fine-tune β-catenin signaling in a manner dependent on the epithelial-mesenchymal transition program.
© 2020 Déjardin et al.
Figures




Comment in
-
Nesprin-2G tension fine-tunes Wnt/β-catenin signaling.J Cell Biol. 2020 Oct 5;219(10):e202009042. doi: 10.1083/jcb.202009042. J Cell Biol. 2020. PMID: 32966550 Free PMC article.

