The risk of respiratory tract infections and interstitial lung disease with interleukin 12/23 and interleukin 23 antagonists in patients with autoimmune diseases: A systematic review and meta-analysis
- PMID: 32791083
- PMCID: PMC7417275
- DOI: 10.1016/j.jaad.2020.08.026
The risk of respiratory tract infections and interstitial lung disease with interleukin 12/23 and interleukin 23 antagonists in patients with autoimmune diseases: A systematic review and meta-analysis
Abstract
Background: Respiratory tract infections (RTIs) and interstitial lung disease (ILD) secondary to interleukin (IL) 12/23 or IL-23 antagonists have been reported in autoimmune diseases.
Objective: To assess the risk of RTIs and noninfectious ILD with these drugs.
Methods: We conducted a systematic review and meta-analysis of randomized controlled trials. Risk of RTIs and noninfectious ILD was compared to placebo by Mantel-Haenszel risk difference. We divided RTIs into upper RTIs (URTI), viral URTIs, and lower RTIs (LRTIs) including infectious pneumonia. Noninfectious ILD included ILD, eosinophilic pneumonia, and pneumonitis.
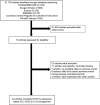
Results: We identified 54 randomized controlled trials including 10,907 patients with 6 IL-12/23 or IL-23 antagonists and 5175 patients with placebo. These drugs significantly increased the risk of RTIs (Mantel-Haenszel risk difference, 0.019; 95% confidence interval, 0.005-0.033; P = .007), which was attributed to URTIs, but not viral URTIs or LRTIs. There was no significant difference in infectious pneumonia and noninfectious ILD between 2 groups.
Limitations: Because of the rarity of infectious pneumonia and ILD, sensitivity analysis was required.
Conclusions: The use of IL-12/23 or IL-23 antagonists for autoimmune diseases increased the risk of URTIs, but not viral URTIs, LRTIs, and noninfectious ILD.
Keywords: IL12/23 and IL23 antagonists; autoimmune diseases; meta-analysis; noninfectious interstitial lung disease; respiratory tract infections.
Copyright © 2020 American Academy of Dermatology, Inc. Published by Elsevier Inc. All rights reserved.
Figures



Comment in
-
Commentary.J Am Acad Dermatol. 2021 Mar;84(3):e161-e162. doi: 10.1016/j.jaad.2020.10.001. Epub 2020 Oct 8. J Am Acad Dermatol. 2021. PMID: 33039482 Free PMC article. No abstract available.
References
-
- Gordon K.B., Langley R.G., Gottlieb A.B., et al. A phase III, randomized, controlled trial of the fully human IL-12/23 mAb briakinumab in moderate-to-severe psoriasis. J Invest Dermatol. 2012;132:304–314. - PubMed
-
- Papp K.A., Langley R.G., Lebwohl M., et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) Lancet. 2008;371:1675–1684. - PubMed
-
- Sandborn W.J., Gasink C., Gao L.L., et al. Ustekinumab induction and maintenance therapy in refractory Crohn's disease. N Engl J Med. 2012;367:1519–1528. - PubMed
-
- Reich K., Papp K.A., Blauvelt A., et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390:276–288. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

