Quantitative Fluxomics of Circulating Metabolites
- PMID: 32791100
- PMCID: PMC7544659
- DOI: 10.1016/j.cmet.2020.07.013
Quantitative Fluxomics of Circulating Metabolites
Abstract
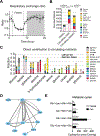
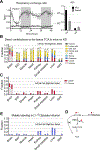
Mammalian organs are nourished by nutrients carried by the blood circulation. These nutrients originate from diet and internal stores, and can undergo various interconversions before their eventual use as tissue fuel. Here we develop isotope tracing, mass spectrometry, and mathematical analysis methods to determine the direct sources of circulating nutrients, their interconversion rates, and eventual tissue-specific contributions to TCA cycle metabolism. Experiments with fifteen nutrient tracers enabled extensive accounting for both circulatory metabolic cycles and tissue TCA inputs, across fed and fasted mice on either high-carbohydrate or ketogenic diet. We find that a majority of circulating carbon flux is carried by two major cycles: glucose-lactate and triglyceride-glycerol-fatty acid. Futile cycling through these pathways is prominent when dietary content of the associated nutrients is low, rendering internal metabolic activity robust to food choice. The presented in vivo flux quantification methods are broadly applicable to different physiological and disease states.
Keywords: TCA cycle; circulating metabolites; energy metabolism; in vivo flux quantification; isotope tracing; ketogenic diet; metabolic cycling.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests J.D.R. is a member of the Rutgers Cancer Institute of New Jersey and of the University of Pennsylvania Diabetes Research Center; a co-founder and stockholder in VL54, Sofro, and Raze Therapeutics; and advisor and stockholder in Agios Pharmaceuticals, Kadmon Pharmaceuticals, Bantam Pharmaceuticals, Colorado Research Partners, Rafael Pharmaceuticals, and L.E.A.F. Pharmaceuticals.
Figures




References
-
- Antoniewicz MR (2015). Methods and advances in metabolic flux analysis: a mini-review. Journal of industrial microbiology & biotechnology 42, 317–325. - PubMed
-
- Bing R, Siegel A, Ungar I, and Gilbert M (1954). Metabolism of the human heart: II. Studies on fat, ketone and amino acid metabolism. The American journal of medicine 16, 504–515. - PubMed

