Lipids in the tumor microenvironment: From cancer progression to treatment
- PMID: 32791170
- PMCID: PMC7674189
- DOI: 10.1016/j.plipres.2020.101055
Lipids in the tumor microenvironment: From cancer progression to treatment
Abstract
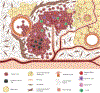
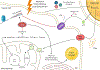
Over the past decade, the study of metabolic abnormalities in cancer cells has risen dramatically. Cancer cells can thrive in challenging environments, be it the hypoxic and nutrient-deplete tumor microenvironment or a distant tissue following metastasis. The ways in which cancer cells utilize lipids are often influenced by the complex interactions within the tumor microenvironment and adjacent stroma. Adipocytes can be activated by cancer cells to lipolyze their triglyceride stores, delivering secreted fatty acids to cancer cells for uptake through numerous fatty acid transporters. Cancer-associated fibroblasts are also implicated in lipid secretion for cancer cell catabolism and lipid signaling leading to activation of mitogenic and migratory pathways. As these cancer-stromal interactions are exacerbated during tumor progression, fatty acids secreted into the microenvironment can impact infiltrating immune cell function and phenotype. Lipid metabolic abnormalities such as increased fatty acid oxidation and de novo lipid synthesis can provide survival advantages for the tumor to resist chemotherapeutic and radiation treatments and alleviate cellular stresses involved in the metastatic cascade. In this review, we highlight recent literature that demonstrates how lipids can shape each part of the cancer lifecycle and show that there is significant potential for therapeutic intervention surrounding lipid metabolic and signaling pathways.
Keywords: Immune Response; Lipid Metabolism; Lipid Signaling; Metastasis; Radiation Therapy; Tumor Microenvironment.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
None declared.
Figures
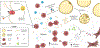
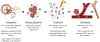
References
-
- Warburg O On the Origin of Cancer Cells. Science (80- ) 1956;123:309–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

