Suvorexant for the prevention of delirium: A meta-analysis
- PMID: 32791676
- PMCID: PMC7386982
- DOI: 10.1097/MD.0000000000021043
Suvorexant for the prevention of delirium: A meta-analysis
Abstract
Background: Delirium is a frequently encountered complication, which is associated with increased mortality. Suvorexant, an approved agent for the treatment of insomnia, is recently suggested to be also effective for prevention of delirium by some authors. However, a consensus has yet to be reached. The goal of this study was to perform a meta-analysis to overall estimate the effectiveness of suvorexant in preventing delirium and its related consequences.
Methods: Eligible studies were identified by searching online databases of PubMed, EMBASE, and Cochrane Library. The pooled OR was calculated for binary outcomes (e.g., the incidence of delirium, mortality, or adverse events), while standardized mean difference (SMD) were expressed for continuous outcomes (e.g., time to delirium onset, length of stay in hospital and ICU, time on ventilation).
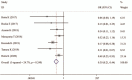
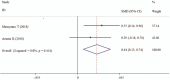
Results: Seven studies which comprised 402 suvorexant treatment patients and 487 patients with control treatment were included in this meta-analysis. Overall, pooled analysis indicated the incidence of delirium could be significantly reduced (OR, 0.30; P < .001) and time to delirium onset was significantly lengthened (SMD, 0.44; P = .006) in patients undergoing suvorexant treatment compared with controls. Suvorexant had no beneficial effects on the secondary outcomes [length of stay in hospital (SMD, -0.65; P = .161) and ICU (SMD, 0.34; P = .297), time on ventilation (SMD, 1.09; P = .318), drug-related adverse events (OR, drug-related adverse events (OR, 1.66; P = .319) and mortality (OR, 2.21; P = .261)]. Subgroup analysis also confirmed the benefit of suvorexant on the development of delirium, which was significant in any subgroup.
Conclusion: Suvorexant should be recommended for the prevention of delirium in clinic.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


References
-
- Mattoo SK, Grover S, Gupta N. Delirium in general practice. Indian J Med Res 2010;131:387–98. - PubMed
-
- Kanova M, Sklienka P, Roman K, et al. Incidence and risk factors for delirium development in ICU patients - a prospective observational study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2017;161:187–96. - PubMed
-
- Aitken SJ, Blyth FM, Naganathan V. Incidence, prognostic factors and impact of postoperative delirium after major vascular surgery: a meta-analysis and systematic review. Vasc Med 2017;22:387–97. - PubMed
-
- Habeeb-Allah A, Alshraideh JA. Delirium post-cardiac surgery: incidence and associated factors. Nurs Crit Care 2019;doi: 10.1111/nicc.12492. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

