Staphylococcal Biofilm Development: Structure, Regulation, and Treatment Strategies
- PMID: 32792334
- PMCID: PMC7430342
- DOI: 10.1128/MMBR.00026-19
Staphylococcal Biofilm Development: Structure, Regulation, and Treatment Strategies
Abstract
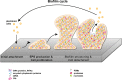
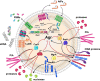
In many natural and clinical settings, bacteria are associated with some type of biotic or abiotic surface that enables them to form biofilms, a multicellular lifestyle with bacteria embedded in an extracellular matrix. Staphylococcus aureus and Staphylococcus epidermidis, the most frequent causes of biofilm-associated infections on indwelling medical devices, can switch between an existence as single free-floating cells and multicellular biofilms. During biofilm formation, cells first attach to a surface and then multiply to form microcolonies. They subsequently produce the extracellular matrix, a hallmark of biofilm formation, which consists of polysaccharides, proteins, and extracellular DNA. After biofilm maturation into three-dimensional structures, the biofilm community undergoes a disassembly process that leads to the dissemination of staphylococcal cells. As biofilms are dynamic and complex biological systems, staphylococci have evolved a vast network of regulatory mechanisms to modify and fine-tune biofilm development upon changes in environmental conditions. Thus, biofilm formation is used as a strategy for survival and persistence in the human host and can serve as a reservoir for spreading to new infection sites. Moreover, staphylococcal biofilms provide enhanced resilience toward antibiotics and the immune response and impose remarkable therapeutic challenges in clinics worldwide. This review provides an overview and an updated perspective on staphylococcal biofilms, describing the characteristic features of biofilm formation, the structural and functional properties of the biofilm matrix, and the most important mechanisms involved in the regulation of staphylococcal biofilm formation. Finally, we highlight promising strategies and technologies, including multitargeted or combinational therapies, to eradicate staphylococcal biofilms.
Keywords: Staphylococcus aureus; Staphylococcus epidermidis; biofilms; extracellular matrix; microbial communities; quorum sensing.
Copyright © 2020 American Society for Microbiology.
Figures
References
-
- Peters G, Locci R, Pulverer G. 1981. Microbial colonization of prosthetic devices. II. Scanning electron microscopy of naturally infected intravenous catheters. Zentralbl Bakteriol Mikrobiol Hyg B 173:293–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

