Empirical validation of a touchscreen probabilistic reward task in rats
- PMID: 32792526
- PMCID: PMC7426406
- DOI: 10.1038/s41398-020-00969-1
Empirical validation of a touchscreen probabilistic reward task in rats
Abstract
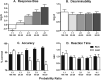
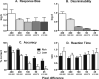
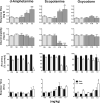
Anhedonia, the loss of pleasure from previously rewarding activities, is implicated in several neuropsychiatric conditions, including major depressive disorder (MDD). In order to accelerate drug development for mood disorders, quantitative approaches are needed to objectively measure responsiveness to reward as a means to identify deficits. One such approach, the probabilistic reward task (PRT), uses visual discrimination methodology to quantify reward learning. In this computerized task, humans make visual discriminations, and probabilistic contingencies are arranged such that correct responses to one alternative are rewarded more often (rich) than correct responses to the other (lean). Healthy participants consistently develop a response bias in favor of the rich alternative. However, participants with MDD typically exhibit lower response biases, and this blunting correlates with current and future anhedonia. The present studies validated a touchscreen-based PRT in rodents with formal and functional similarity to the human task. First, rats were trained to discriminate between two lines that differed in length. Next, parametric manipulations of probabilistic contingencies, line-length stimuli, and drug treatment (amphetamine, 0.32-3.2 mg/kg; scopolamine, 0.1-1.0 mg/kg; oxycodone, 0.1-1.0 mg/kg) on response bias were evaluated. Results demonstrated orderly shifts in bias and discriminability that varied as a function of, respectively, the asymmetry of rich/lean probabilities and disparity in line lengths. Drugs that enhance reward responsiveness (amphetamine and scopolamine, but not oxycodone) increased bias, verifying pharmacological task sensitivity. Finally, performance outcomes under optimized conditions were replicated in female rats. Collectively, the touchscreen-based rodent PRT appears to have high preclinical value as a quantitative assay of reward learning.
Conflict of interest statement
Over the past 3 years, D.A.P. has received funding from NIMH, Brain and Behavior Research Foundation, the Dana Foundation, and Millennium Pharmaceuticals; consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Boehreinger Ingelheim, Compass Pathway, Posit Science, Otsuka Pharmaceuticals, and Takeda Pharmaceuticals; one honorarium from Alkermes; stock options from BlackThorn Therapeutics. D.A.P. has a financial interest in BlackThorn Therapeutics, which has licensed the copyright to the Probabilistic Reward Task through Harvard University. D.A.P.’s interests were reviewed and are managed by McLean Hospital and Partners HealthCare in accordance with their conflict of interest policies. All other authors report no biomedical financial interests or potential conflicts of interest.
Figures


References
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edn. Arlington: American Psychiatric Association Publishing; 2013.
-
- World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines (World Health Organization, Geneva, Switzerland, 1992).
-
- Klein DF. Endogenomorphic depression—conceptual and terminological revision. Arch. Gen. Psychiatry. 1974;31:447–454. - PubMed
-
- Leibenluft E, Charney DS, Pine DS. Researching the pathophysiology of pediatric bipolar disorder. Biol. Psychiatry. 2003;53:1009–1020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH101521/MH/NIMH NIH HHS/United States
- UH2 MH109334/MH/NIMH NIH HHS/United States
- UH2-MH109334/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)/International
- K01-DA035974/U.S. Department of Health & Human Services | NIH | National Institute on Drug Abuse (NIDA)/International
- R37 MH068376/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

