Evaluation of Biased and Balanced Salvinorin A Analogs in Preclinical Models of Pain
- PMID: 32792903
- PMCID: PMC7385413
- DOI: 10.3389/fnins.2020.00765
Evaluation of Biased and Balanced Salvinorin A Analogs in Preclinical Models of Pain
Abstract
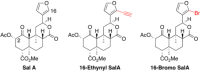
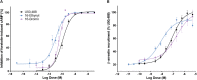
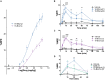
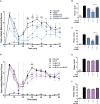
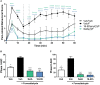
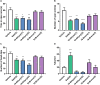
In the search for safer, non-addictive analgesics, kappa opioid receptor (KOPr) agonists are a potential target, as unlike mu-opioid analgesics, they do not have abuse potential. Salvinorin A (SalA) is a potent and selective KOPr agonist, however, clinical utility is limited by the short duration of action and aversive side effects. Biasing KOPr signaling toward G-protein activation has been highlighted as a key cellular mechanism to reduce the side effects of KOPr agonists. The present study investigated KOPr signaling bias and the acute antinociceptive effects and side effects of two novel analogs of SalA, 16-Bromo SalA and 16-Ethynyl SalA. 16-Bromo SalA showed G-protein signaling bias, whereas 16-Ethynyl SalA displayed balanced signaling properties. In the dose-response tail-withdrawal assay, SalA, 16-Ethynyl SalA and 16-Bromo SalA were more potent than the traditional KOPr agonist U50,488, and 16-Ethynyl SalA was more efficacious. 16-Ethynyl SalA and 16-Bromo SalA both had a longer duration of action in the warm water tail-withdrawal assay, and 16-Ethynyl had greater antinociceptive effect in the hot-plate assay, compared to SalA. In the intraplantar 2% formaldehyde test, 16-Ethynyl SalA and 16-Bromo SalA significantly reduced both nociceptive and inflammatory pain-related behaviors. Moreover, 16-Ethynyl SalA and 16-Bromo SalA had no anxiogenic effects in the marble burying task, and 16-Bromo SalA did not alter behavior in the elevated zero maze. Overall, 16-Ethynyl SalA significantly attenuated acute pain-related behaviors in multiple preclinical models, while the biased KOPr agonist, 16-Bromo SalA, displayed modest antinociceptive effects, and lacked anxiogenic effects.
Keywords: Salvinorin A; antinociception; anxiety; biased agonism; kappa opioid receptor.
Copyright © 2020 Paton, Biggerstaff, Kaska, Crowley, La Flamme, Prisinzano and Kivell.
Figures

References
-
- Aviello G., Borrelli F., Guida F., Romano B., Lewellyn K., De Chiaro M., et al. (2011). Ultrapotent effects of salvinorin A, a hallucinogenic compound from Salvia divinorum, on LPS-stimulated murine macrophages and its anti-inflammatory action in vivo. J. Mol. Med. 89 891–902. 10.1007/s00109-011-0752-4 - DOI - PubMed
-
- Bals-Kubik R., Ableitner A., Herz A., Shippenberg T. S. (1993). Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J. Pharmacol. Exp. Ther. 264 489–495. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

