Genetics of Hypertriglyceridemia
- PMID: 32793115
- PMCID: PMC7393009
- DOI: 10.3389/fendo.2020.00455
Genetics of Hypertriglyceridemia
Abstract
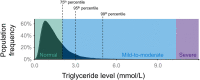
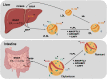
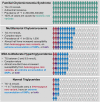
Hypertriglyceridemia, a commonly encountered phenotype in cardiovascular and metabolic clinics, is surprisingly complex. A range of genetic variants, from single-nucleotide variants to large-scale copy number variants, can lead to either the severe or mild-to-moderate forms of the disease. At the genetic level, severely elevated triglyceride levels resulting from familial chylomicronemia syndrome (FCS) are caused by homozygous or biallelic loss-of-function variants in LPL, APOC2, APOA5, LMF1, and GPIHBP1 genes. In contrast, susceptibility to multifactorial chylomicronemia (MCM), which has an estimated prevalence of ~1 in 600 and is at least 50-100-times more common than FCS, results from two different types of genetic variants: (1) rare heterozygous variants (minor allele frequency <1%) with variable penetrance in the five causal genes for FCS; and (2) common variants (minor allele frequency >5%) whose individually small phenotypic effects are quantified using a polygenic score. There is indirect evidence of similar complex genetic predisposition in other clinical phenotypes that have a component of hypertriglyceridemia, such as combined hyperlipidemia and dysbetalipoproteinemia. Future considerations include: (1) evaluation of whether the specific type of genetic predisposition to hypertriglyceridemia affects medical decisions or long-term outcomes; and (2) searching for other genetic contributors, including the role of genome-wide polygenic scores, novel genes, non-linear gene-gene or gene-environment interactions, and non-genomic mechanisms including epigenetics and mitochondrial DNA.
Keywords: autosomal recessive; complex trait; familial chylomicronemia syndrome (FCS); multifactoriel chylomicronemia (MCM); polygenic score; triglyceride.
Copyright © 2020 Dron and Hegele.
Figures
References
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

