The Long Road Toward COVID-19 Herd Immunity: Vaccine Platform Technologies and Mass Immunization Strategies
- PMID: 32793245
- PMCID: PMC7385234
- DOI: 10.3389/fimmu.2020.01817
The Long Road Toward COVID-19 Herd Immunity: Vaccine Platform Technologies and Mass Immunization Strategies
Abstract
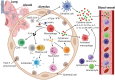
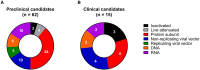
There is an urgent need for effective countermeasures against the current emergence and accelerating expansion of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Induction of herd immunity by mass vaccination has been a very successful strategy for preventing the spread of many infectious diseases, hence protecting the most vulnerable population groups unable to develop immunity, for example individuals with immunodeficiencies or a weakened immune system due to underlying medical or debilitating conditions. Therefore, vaccination represents one of the most promising counter-pandemic measures to COVID-19. However, to date, no licensed vaccine exists, neither for SARS-CoV-2 nor for the closely related SARS-CoV or Middle East respiratory syndrome-CoV. In addition, a few vaccine candidates have only recently entered human clinical trials, which hampers the progress in tackling COVID-19 infection. Here, we discuss potential prophylactic interventions for SARS-CoV-2 with a focus on the challenges existing for vaccine development, and we review pre-clinical progress and ongoing human clinical trials of COVID-19 vaccine candidates. Although COVID-19 vaccine development is currently accelerated via so-called fast-track programs, vaccines may not be timely available to have an impact on the first wave of the ongoing COVID-19 pandemic. Nevertheless, COVID-19 vaccines will be essential in the future for reducing morbidity and mortality and inducing herd immunity, if SARS-CoV-2 becomes established in the population like for example influenza virus.
Keywords: COVID-19; SARS-CoV-2; animal models; coronavirus; herd immunity; immune response; immunopathology; vaccine.
Copyright © 2020 Frederiksen, Zhang, Foged and Thakur.
Figures

References
-
- WHO . Coronavirus Disease 2019 (COVID-19): Situation Report, 155. 2020. (2020). Available online at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/2... (accessed June 23, 2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

