Influence of the MUC1 Cell Surface Mucin on Gastric Mucosal Gene Expression Profiles in Response to Helicobacter pylori Infection in Mice
- PMID: 32793510
- PMCID: PMC7393270
- DOI: 10.3389/fcimb.2020.00343
Influence of the MUC1 Cell Surface Mucin on Gastric Mucosal Gene Expression Profiles in Response to Helicobacter pylori Infection in Mice
Abstract
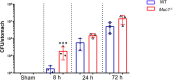
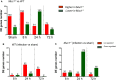
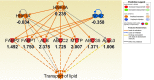
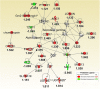
The cell surface mucin MUC1 is an important host factor limiting Helicobacter pylori (H. pylori) pathogenesis in both humans and mice by providing a protective barrier and modulating mucosal epithelial and leukocyte responses. The aim of this study was to establish the time-course of molecular events in MUC1-modulated gene expression profiles in response to H. pylori infection in wild type (WT) and MUC1-deficient mice using microarray-determined mRNA expression, gene network analysis and Ingenuity Pathway Analysis (IPA). A time-course over the first 72 h of infection showed significantly higher mucosal loads of bacteria at 8 h of infection in Muc1-/- mice compared with WT, confirming its importance in the early stages of infection (P = 0.0003). Microarray analysis revealed 266 differentially expressed genes at one or more time-points over 72 h in the gastric mucosa of Muc1-/- mice compared with WT control using a threshold of 2-fold change. The SPINK1 pancreatic cancer canonical pathway was strongly inhibited in Muc1-/- mice compared with WT at sham and 8 h infection (P = 6.08E-14 and P = 2.25 E-19, respectively) but potently activated at 24 and 72 h post-infection (P = 1.38E-22 and P = 5.87E-13, respectively). The changes in this pathway are reflective of higher expression of genes mediating digestion and absorption of lipids, carbohydrates, and proteins at sham and 8 h infection in the absence of MUC1, but that this transcriptional signature is highly down regulated as infection progresses in the absence of MUC1. Uninfected Muc1-/- gastric tissue was highly enriched for expression of factors involved in lipid metabolism and 8 h infection further activated this network compared with WT. As infection progressed, a network of antimicrobial and anti-inflammatory response genes was more highly activated in Muc1-/- than WT mice. Key target genes identified by time-course microarrays were independently validated using RT-qPCR. These results highlight the dynamic interplay between the host and H. pylori, and the role of MUC1 in host defense, and provide a general picture of changes in cellular gene expression modulated by MUC1 in a time-dependent manner in response to H. pylori infection.
Keywords: Helicobacter pylori; MUC1; SPINK1; gene expression; infection; lipid metabolism.
Copyright © 2020 Sheng, Ng, Summers, Every, Price, Hasnain, Sutton and McGuckin.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

