Recent Advances on Drug-Loaded Mesenchymal Stem Cells With Anti-neoplastic Agents for Targeted Treatment of Cancer
- PMID: 32793565
- PMCID: PMC7390947
- DOI: 10.3389/fbioe.2020.00748
Recent Advances on Drug-Loaded Mesenchymal Stem Cells With Anti-neoplastic Agents for Targeted Treatment of Cancer
Abstract
Mesenchymal stem cells (MSCs), as an undifferentiated group of adult multipotent cells, have remarkable antitumor features that bring them up as a novel choice to treat cancers. MSCs are capable of altering the behavior of cells in the tumor microenvironment, inducing an anti-inflammatory effect in tumor cells, inhibiting tumor angiogenesis, and preventing metastasis. Besides, MSCs can induce apoptosis and inhibit the proliferation of tumor cells. The ability of MSCs to be loaded with chemotherapeutic drugs and release them in the site of primary and metastatic neoplasms makes them a preferable choice as targeted drug delivery procedure. Targeted drug delivery minimizes unexpected side effects of chemotherapeutic drugs and improves clinical outcomes. This review focuses on recent advances on innate antineoplastic features of MSCs and the effect of chemotherapeutic drugs on viability, proliferation, and the regenerative capacity of various kinds of MSCs. It also discusses the efficacy and mechanisms of drug loading and releasing procedures along with in vivo and in vitro preclinical outcomes of antineoplastic effects of primed MSCs for clinical prospection.
Keywords: angiogenesis; apoptosis; cancer; chemotherapeutic drugs; mesenchymal stem cell; metastasis; proliferation; targeted therapy.
Copyright © 2020 Babajani, Soltani, Jamshidi, Farjoo and Niknejad.
Figures
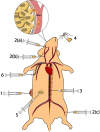
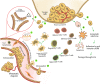
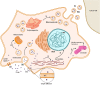
References
-
- Ahn J. O., Chae J. S., Coh Y. R., Jung W. S., Lee H. W., Shin I. S., et al. (2014). Human adipose tissue-derived mesenchymal stem cells inhibit T-cell lymphoma growth in vitro and in vivo. Anticancer Res. 34 4839– 4847. - PubMed
-
- Akimoto K., Kimura K., Nagano M., Takano S., To’a Salazar G., Yamashita T., et al. (2013). Umbilical cord blood-derived mesenchymal stem cells inhibit, but adipose tissue-derived mesenchymal stem cells promote, glioblastoma multiforme proliferation. Stem Cells Dev. 22 1370–1386. 10.1089/scd.2012.0486 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

