Cancer-associated fibroblasts promote the migration and invasion of gastric cancer cells via activating IL-17a/JAK2/STAT3 signaling
- PMID: 32793721
- PMCID: PMC7396760
- DOI: 10.21037/atm-20-4843
Cancer-associated fibroblasts promote the migration and invasion of gastric cancer cells via activating IL-17a/JAK2/STAT3 signaling
Abstract
Background: Cancer-associated fibroblasts (CAFs), as the activated stroma cells, contribute to tumor progression via the release of cytokines, growth factors, and hormones. However, neither the factors produced by CAFs nor the molecular mechanisms were illuminated very well in gastric cancer (GC).
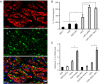
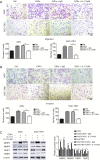
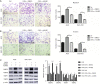
Methods: Immunohistochemical staining of alpha-smooth muscle actin (α-SMA) was applied to examine the number of CAFs in GC samples from 227 patients. ELISA and qRT-PCR were performed to detect the expression of interleukin 17a (IL-17a). The migration and invasion of GC cells were determined by the Transwell assay. The expressions of JAK2, STAT3, MMP-2, MMP-9, TIMP-1, and TIMP-2 were measured by western blotting. IL-17a was blocked with a polyclonal antibody, and JAK2/STAT3 signaling pathway was blocked by a specific inhibitor AG490.
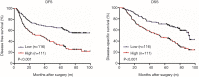
Results: High CAFs in GC tissues were positively correlated with advanced TNM stage and perineural invasion. Furthermore, GC patients with high CAFs in tumor tissues had an obvious worse disease-free survival (DFS) and disease-special survival (DSS). Multivariate analysis showed that high CAFs in GC tissues were an independent risk factor for DFS and DSS. CAFs expressed IL-17a significantly after GC cell co-culture. CAFs markedly enhanced the migration and invasion abilities of AGS and SGC-7901 cells. Moreover, CAFs co-culture resulted in increased levels of MMP2/9, reduced expressions of TIMP1/2, and activation of the JAK2/STAT3 signaling pathway in the GC cells. IL-17a neutralizing antibody or JAK2 inhibitor AG490 can significantly inhibit the effects of CAFs on the migration, invasion, MMP2/9, TIMP1/2, and JAK2/STAT3 pathways of GC cells.
Conclusions: CAFs correlated with unfavorable clinical features and poor prognosis of GC patients. CAFs secreted IL-17a, which promoted the migration and invasion of GC cells through activating JAK2/STAT3 signaling. These results may identify IL-17a as a promising prognostic marker and therapeutic target of GC.
Keywords: Gastric cancer (GC); IL-17a; cancer-associated fibroblasts (CAFs); prognosis; tumor metastasis.
2020 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4843). The authors have no conflicts of interest to declare.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
