This is a preprint.
Robotic High-Throughput Biomanufacturing and Functional Differentiation of Human Pluripotent Stem Cells
- PMID: 32793899
- PMCID: PMC7418713
- DOI: 10.1101/2020.08.03.235242
Robotic High-Throughput Biomanufacturing and Functional Differentiation of Human Pluripotent Stem Cells
Update in
-
Robotic high-throughput biomanufacturing and functional differentiation of human pluripotent stem cells.Stem Cell Reports. 2021 Dec 14;16(12):3076-3092. doi: 10.1016/j.stemcr.2021.11.004. Epub 2021 Dec 2. Stem Cell Reports. 2021. PMID: 34861164 Free PMC article.
Abstract
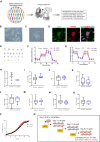
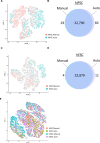
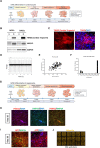
Efficient translation of human induced pluripotent stem cells (hiPSCs) depends on implementing scalable cell manufacturing strategies that ensure optimal self-renewal and functional differentiation. Currently, manual culture of hiPSCs is highly variable and labor-intensive posing significant challenges for high-throughput applications. Here, we established a robotic platform and automated all essential steps of hiPSC culture and differentiation under chemically defined conditions. This streamlined approach allowed rapid and standardized manufacturing of billions of hiPSCs that can be produced in parallel from up to 90 different patient-and disease-specific cell lines. Moreover, we established automated multi-lineage differentiation to generate primary embryonic germ layers and more mature phenotypes such as neurons, cardiomyocytes, and hepatocytes. To validate our approach, we carefully compared robotic and manual cell culture and performed molecular and functional cell characterizations (e.g. bulk culture and single-cell transcriptomics, mass cytometry, metabolism, electrophysiology, Zika virus experiments) in order to benchmark industrial-scale cell culture operations towards building an integrated platform for efficient cell manufacturing for disease modeling, drug screening, and cell therapy. Combining stem cell-based models and non-stop robotic cell culture may become a powerful strategy to increase scientific rigor and productivity, which are particularly important during public health emergencies (e.g. opioid crisis, COVID-19 pandemic).
Conflict of interest statement
COMPETING INTERESTS
The authors declare no competing interests.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
