This is a preprint.
Structural analysis of full-length SARS-CoV-2 spike protein from an advanced vaccine candidate
- PMID: 32793901
- PMCID: PMC7418715
- DOI: 10.1101/2020.08.06.234674
Structural analysis of full-length SARS-CoV-2 spike protein from an advanced vaccine candidate
Update in
-
Structural analysis of full-length SARS-CoV-2 spike protein from an advanced vaccine candidate.Science. 2020 Nov 27;370(6520):1089-1094. doi: 10.1126/science.abe1502. Epub 2020 Oct 20. Science. 2020. PMID: 33082295 Free PMC article.
Abstract
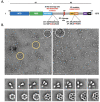
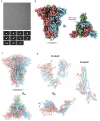
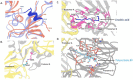
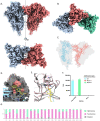
Vaccine efforts against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) responsible for the current COVID-19 pandemic are focused on SARS-CoV-2 spike glycoprotein, the primary target for neutralizing antibodies. Here, we performed cryo-EM and site-specific glycan analysis of one of the leading subunit vaccine candidates from Novavax based on a full-length spike protein formulated in polysorbate 80 (PS 80) detergent. Our studies reveal a stable prefusion conformation of the spike immunogen with slight differences in the S1 subunit compared to published spike ectodomain structures. Interestingly, we also observed novel interactions between the spike trimers allowing formation of higher order spike complexes. This study confirms the structural integrity of the full-length spike protein immunogen and provides a basis for interpreting immune responses to this multivalent nanoparticle immunogen.
Conflict of interest statement
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
