Effect of biofilm exposure on marginal integrity of composite restorations
- PMID: 32794395
- PMCID: PMC8136684
Effect of biofilm exposure on marginal integrity of composite restorations
Abstract
Purpose: To evaluate the effect of bacterial exposure on the marginal integrity of dentin-resin interfaces for composites with and without bioactive glass (BAG).
Methods: Cavity preparations of 5 mm width and 1.5 mm depth were machined into dentin disks by means of a computer controlled milling system. After applying the bonding agent, cavity preparations (n=3-5) were restored by incremental technique with experimental resin composites (50:50 BisGMA/TEGDMA: 72wt% filler) with different filler compositions: control - 67 wt% silanated strontium glass and 5wt% aerosol-silica filler and BAG - 57 wt% silanated strontium glass and 15 wt% BAG-65 wt% silica. Samples were then stored in sterile Todd-Hewitt media or co-incubated with Streptococcus mutans (UA 159), at 37°C, 5% CO2 for 1-2 weeks. For samples co-incubated with a living biofilm, a luciferase assay was performed in order to assess its viability. Surfaces were impressed before and after each storage condition and replicas examined in a scanning electron microscope. Using image analysis software (Image J), the discontinuous margins percentage (%DM) was quantitatively assessed. Data were analyzed using two-way ANOVA followed by Tukey's test (α= 0.05).
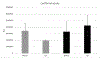
Results: Gap size ranged between 7-23 µm. The bacterial exposure significantly increased the %DM in both groups predominantly due to the formation of new gap regions. There was no difference between control and BAG composites regarding %DM and the biofilm viability. Bacterial exposure promoted degradation of composite restoration marginal integrity, with no difference between composites with and without BAG.
Clinical significance: The samples incubated with living biofilm had a higher gap percentage in the margins, confirming the negative effect of cariogenic bacteria on margin degradation. The parameters defined for such synergy can help to understand the multi-factorial aspect of marginal discontinuity and therefore, predict the behavior of composite restorations subjected to the challenging oral environment.
Copyright©American Journal of Dentistry.
Conflict of interest statement
The authors declared no conflict of interest. This work was supported by the Brazilian Federal Agency for Support and Evaluation of Graduate Education - CAPES and funded with public resources obtained through the "Public Call 15/2017" - Fundação Araucária/Secretaria de Estado da Ciência, Tecnologia e Ensino Superior do Paraná (SETI), grant #15.561.047-6.
Figures



References
-
- Delaviz Y, Finer Y, Santerre JP. Biodegradation of resin composites and adhesives by oral bacteria and saliva: A rationale for new material designs that consider the clinical environment and treatment challenges. Dent Mater 2014;30:16–32. - PubMed
-
- Krämer N, Schmidt M, Lücker S, Domann E, Frankenberger R. Glass ionomer cement inhibits secondary caries in an in vitro biofilm model. Clin Oral Investig 2018;22:1019–1031. - PubMed
-
- Lopes MB, Costa LA, Consani S, Gonini AJ, Sinhoreti MA. SEM evaluation of marginal sealing on composite restorations using different photoactivation and composite insertion methods. Indian J Dent Res 2009;20:394–399. - PubMed
-
- Naumova EA, Ernst S, Schaper K, Arnold WH, Piwowarczyk A. Adhesion of different resin cements to enamel and dentin. Dent Mater J 2016;35:345–352. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
