A Notch3-Marked Subpopulation of Vascular Smooth Muscle Cells Is the Cell of Origin for Occlusive Pulmonary Vascular Lesions
- PMID: 32794408
- PMCID: PMC7578108
- DOI: 10.1161/CIRCULATIONAHA.120.045750
A Notch3-Marked Subpopulation of Vascular Smooth Muscle Cells Is the Cell of Origin for Occlusive Pulmonary Vascular Lesions
Abstract
Background: Pulmonary arterial hypertension (PAH) is a fatal disease characterized by profound vascular remodeling in which pulmonary arteries narrow because of medial thickening and occlusion by neointimal lesions, resulting in elevated pulmonary vascular resistance and right heart failure. Therapies targeting the neointima would represent a significant advance in PAH treatment; however, our understanding of the cellular events driving neointima formation, and the molecular pathways that control them, remains limited.
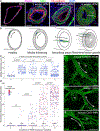
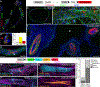
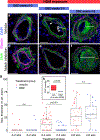
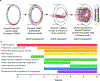
Methods: We comprehensively map the stepwise remodeling of pulmonary arteries in a robust, chronic inflammatory mouse model of pulmonary hypertension. This model demonstrates pathological features of the human disease, including increased right ventricular pressures, medial thickening, neointimal lesion formation, elastin breakdown, increased anastomosis within the bronchial circulation, and perivascular inflammation. Using genetic lineage tracing, clonal analysis, multiplexed in situ hybridization, immunostaining, deep confocal imaging, and staged pharmacological inhibition, we define the cell behaviors underlying each stage of vascular remodeling and identify a pathway required for neointima formation.
Results: Neointima arises from smooth muscle cells (SMCs) and not endothelium. Medial SMCs proliferate broadly to thicken the media, after which a small number of SMCs are selected to establish the neointima. These neointimal founder cells subsequently undergoing massive clonal expansion to form occlusive neointimal lesions. The normal pulmonary artery SMC population is heterogeneous, and we identify a Notch3-marked minority subset of SMCs as the major neointimal cell of origin. Notch signaling is specifically required for the selection of neointimal founder cells, and Notch inhibition significantly improves pulmonary artery pressure in animals with pulmonary hypertension.
Conclusions: This work describes the first nongenetically driven murine model of pulmonary hypertension (PH) that generates robust and diffuse occlusive neointimal lesions across the pulmonary vascular bed and does so in a stereotyped timeframe. We uncover distinct cellular and molecular mechanisms underlying medial thickening and neointima formation and highlight novel transcriptional, behavioral, and pathogenic heterogeneity within pulmonary artery SMCs. In this model, inflammation is sufficient to generate characteristic vascular pathologies and physiological measures of human PAH. We hope that identifying the molecular cues regulating each stage of vascular remodeling will open new avenues for therapeutic advancements in the treatment of PAH.
Keywords: cell lineage; clones; inflammation; neointima; pulmonary hypertension; vascular smooth muscle cells.
Figures




References
-
- Humbert M, Guignabert C, Bonnet S, Dorfmuller P, Klinger JR, Nicolls MR, Olschewski AJ, Pullamsetti SS, Schermuly RT, Stenmark KR, et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J. 2019;53:1801887. doi: 10.1183/13993003.01887-2018. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

