The iboga enigma: the chemistry and neuropharmacology of iboga alkaloids and related analogs
- PMID: 32794540
- PMCID: PMC7882011
- DOI: 10.1039/d0np00033g
The iboga enigma: the chemistry and neuropharmacology of iboga alkaloids and related analogs
Abstract
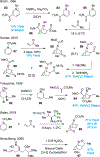
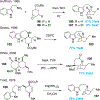
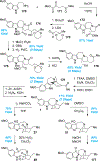
Covering: 2000 up to 2020 Few classes of natural products have inspired as many chemists and biologists as have the iboga alkaloids. This family of monoterpenoid indole alkaloids includes the anti-addictive compound ibogaine as well as catharanthine, a precursor to the chemotherapeutic vinblastine. Despite being known for over 120 years, these small molecules continue to challenge our assumptions about biosynthetic pathways, catalyze our creativity for constructing complex architectures, and embolden new approaches for treating mental illness. This review will cover recent advances in both the biosynthesis and chemical synthesis of iboga alkaloids as well as their use as next-generation neurotherapeutics. Whenever appropriate, we provide historical context for the discoveries of the past decade and indicate areas that have yet to be resolved. While significant progress regarding their chemistry and pharmacology has been made since the 1960s, it is clear that the iboga alkaloids will continue to stoke scientific innovation for years to come.
Figures


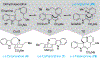
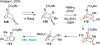
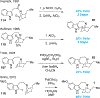
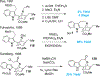
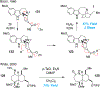
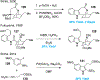
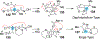

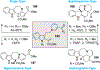

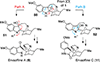
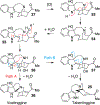

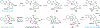
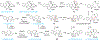
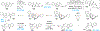

References
-
- Dybowski J, Landrin E. Concerning Iboga, its excitement-producing properties, its composition, and the new alkaloid it contains, ibogaine. CR Acad. Sci 1901; 133:748.
-
- Bartlett MF, Dickel DF, Taylor WI. The alkaloids of Tabernanthe iboga. Part IV. 1 The structures of ibogamine, ibogaine, tabernanthine and voacangine. J. Am. Chem. Soc, 1958, 80, 126–136.
-
- Arai G, Coppola J, and Jeffrey G The Structure of Ibogaine. Acta Crystallogr., 1960, 13, 553–564.
-
- Men JL; Taylor WI A uniform numbering system for indole Alkaloids. Experientia, 1965, 21, 508–510. - PubMed
-
- Martino E; Casamassima G; Castiglione S; Cellupica E; Pantalone S; Papagni F; Rui M; Siciliano AM; Collina S Vinca alkaloids and analogues as anti-cancer agents: Looking back, peering ahead. Bioorganic & Medicinal Chemistry Letters, 2018, 28, 2816–2826. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

