Benzylic Methylene Functionalizations of Diarylmethanes
- PMID: 32794651
- PMCID: PMC7436909
- DOI: 10.1002/asia.202000730
Benzylic Methylene Functionalizations of Diarylmethanes
Abstract
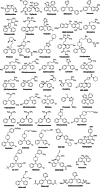
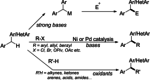
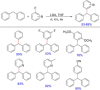
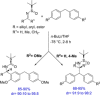
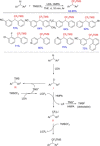
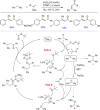
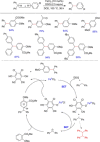
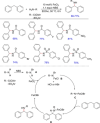
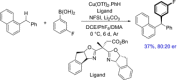
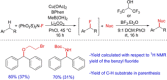
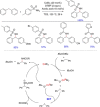
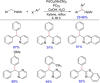
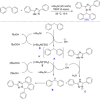
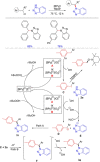
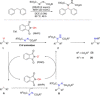
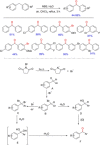
Diarylmethanes are cardinal scaffolds by virtue of their unique structural feature including the presence of a benzylic CH2 group that can be easily functionalized to generate a variety of fascinating molecules holding immense importance in pharmaceutical, agrochemical, and material sciences. While the originally developed protocols for benzylic C-H functionalization in diarylmethanes employing base-mediated and metal-catalyzed strategies are still actively used, they are joined by a new array of metal-free conditions, offering milder and benign conditions. With the recent surge of interest towards the synthesis of functionalized diarylmethanes, numerous choices are now available for a synthetic organic chemist to transform the benzylic C-H bond to C-C or C-X bond offering the synthesis of any molecule of choice. This review highlights benzylic methylene (CH2 ) functionalizations of diaryl/heteroarylmethanes utilizing various base-mediated, transition-metal-catalyzed, and transition-metal free approaches for the synthesis of structurally diverse important organic molecules, often with a high chemo-, regio- and enantio-selectivity. This review also attempts to provide analysis of the scope and limitations, mechanistic understanding, and sustainability of the transformations.
Keywords: Benzylic C−H functionalization; Diarylmethanes.
© 2020 Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



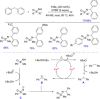
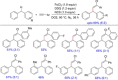



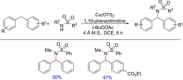

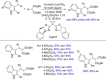


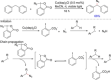
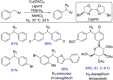
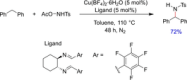
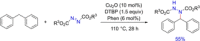

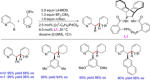
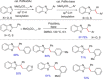


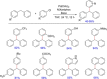
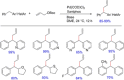
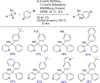
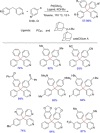

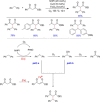
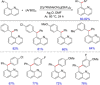
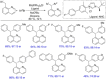
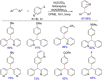
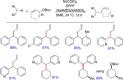
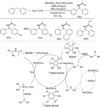
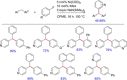
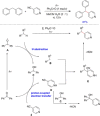
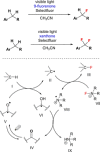

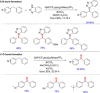




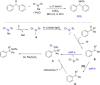
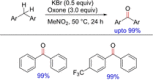



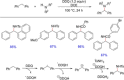
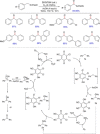


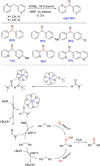
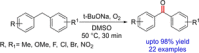
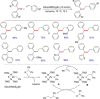
References
-
- None
-
- Nair V., Thomas S., Mathew S. C., Abhilash K. G., Tetrahedron 2006, 62, 6731–6747;
-
- Bolm C., Schmidt F., Stemmler R. T., Rudolph J., Chem. Soc. Rev. 2006, 35, 454–470; - PubMed
-
- Snape T. J., Ameen D., MedChemComm 2013, 4, 893–907;
-
- Rachwalski M., Wujkowska Z., Jarzyński S., Pieczonka A. M., Leśniak S., Tetrahedron: Asymmetry 2016, 27, 1238–1244;
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

