Cangrelor, Tirofiban, and Chewed or Standard Prasugrel Regimens in Patients With ST-Segment-Elevation Myocardial Infarction: Primary Results of the FABOLUS-FASTER Trial
- PMID: 32795098
- PMCID: PMC7392586
- DOI: 10.1161/CIRCULATIONAHA.120.046928
Cangrelor, Tirofiban, and Chewed or Standard Prasugrel Regimens in Patients With ST-Segment-Elevation Myocardial Infarction: Primary Results of the FABOLUS-FASTER Trial
Erratum in
-
Correction to: Cangrelor, Tirofiban, and Chewed or Standard Prasugrel Regimens in Patients With ST-Segment-Elevation Myocardial Infarction: Primary Results of the FABOLUS-FASTER Trial.Circulation. 2020 Aug 4;142(5):e71. doi: 10.1161/CIR.0000000000000910. Epub 2020 Aug 3. Circulation. 2020. PMID: 33210944 Free PMC article. No abstract available.
Abstract
Background: Standard administration of newer oral P2Y12 inhibitors, including prasugrel or ticagrelor, provides suboptimal early inhibition of platelet aggregation (IPA) in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention. We aimed to investigate the effects of cangrelor, tirofiban, and prasugrel, administered as chewed or integral loading dose, on IPA in patients undergoing primary percutaneous coronary intervention.
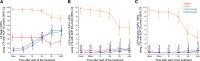
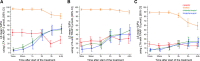
Methods: The FABOLUS-FASTER trial (Facilitation Through Aggrastat or Cangrelor Bolus and Infusion Over Prasugrel: A Multicenter Randomized Open-Label Trial in Patients with ST-Elevation Myocardial Infarction Referred for Primary Percutaneous Intervention) is an investigator-initiated, multicenter, open-label, randomized study. A total of 122 P2Y12-naive patients with ST-segment-elevation myocardial infarction were randomly allocated (1:1:1) to cangrelor (n=40), tirofiban (n=40) (both administered as bolus and 2-hour infusion followed by 60 mg of prasugrel), or 60-mg loading dose of prasugrel (n=42). The latter group underwent an immediate 1:1 subrandomization to chewed (n=21) or integral (n=21) tablets administration. The trial was powered to test 3 hypotheses (noninferiority of cangrelor compared with tirofiban using a noninferiority margin of 9%, superiority of both tirofiban and cangrelor compared with chewed prasugrel, and superiority of chewed prasugrel as compared with integral prasugrel, each with α=0.016 for the primary end point, which was 30-minute IPA at light transmittance aggregometry in response to 20 μmol/L adenosine diphosphate.
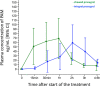
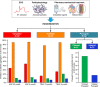
Results: At 30 minutes, cangrelor did not satisfy noninferiority compared with tirofiban, which yielded superior IPA over cangrelor (95.0±8.9 versus 34.1±22.5; P<0.001). Cangrelor or tirofiban were both superior to chewed prasugrel (IPA, 10.5±11.0; P<0.001 for both comparisons), which did not provide higher IPA over integral prasugrel (6.3±11.4; P=0.47), despite yielding higher prasugrel active metabolite concentration (ng/mL; 62.3±82.6 versus 17.1±43.5; P=0.016).
Conclusions: Cangrelor provided inferior IPA compared with tirofiban; both treatments yielded greater IPA compared with chewed prasugrel, which led to higher active metabolite concentration but not greater IPA compared with integral prasugrel. Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT02978040; URL: https://www.clinicaltrialsregister.eu; EudraCT 2017-001065-24.
Keywords: cangrelor; percutaneous coronary intervention; platelet aggregation; prasugrel hydrochloride; tirofiban.
Conflict of interest statement
Dr Gargiulo reports consultant fees from Daiichi-Sankyo outside the submitted work. Dr Gragnano reports research grant support from the European Society of Cardiology. Dr Vranckx reports personal fees from Daiichi-Sankyo, AstraZeneca, Bayer Health Care, and Terumo outside the submitted work. Dr Leonardi reports personal fees from Bayer, Bristol-Myers Squibb SA, Chiesi, Daiichi-Sankyo, and AstraZeneca outside the submitted work. Dr Windecker reports research and educational grants to the institution from Abbott, Amgen, Bayer, BMS, Biotronik, Boston Scientific, CSL Behring, Edwards Lifesciences, Medtronic, Polares, and Sinomed outside the submitted work. Dr Valgimigli reports a grant to the institution from Medicure and grants and personal fees from Abbott, Alvimedica, Amgen, Bayer, Bristol-Myers Squibb SA, Coreflow, Daiichi-Sankyo, Vifor, Idorsia, Terumo, iVascular, and AstraZeneca outside the submitted work. The other authors report no disclosures.
Figures

Comment in
-
Letter by Angiolillo et al Regarding Article, "Cangrelor, Tirofiban, and Chewed or Standard Prasugrel Regimens in Patients With ST-Segment-Elevation Myocardial Infarction: Primary Results of the FABOLUS FASTER Trial".Circulation. 2021 Mar 30;143(13):e795-e796. doi: 10.1161/CIRCULATIONAHA.120.050205. Epub 2021 Mar 29. Circulation. 2021. PMID: 33779271 No abstract available.
-
Response by Gargiulo et al to Letter Regarding Article, "Cangrelor, Tirofiban, and Chewed or Standard Prasugrel Regimens in Patients With ST-Segment-Elevation Myocardial Infarction: Primary Results of the FABOLUS FASTER Trial".Circulation. 2021 Mar 30;143(13):e797-e798. doi: 10.1161/CIRCULATIONAHA.120.051945. Epub 2021 Mar 29. Circulation. 2021. PMID: 33779272 No abstract available.
References
-
- Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, et al. ; ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 201839119–177doi: 10.1093/eurheartj/ehx393 - PubMed
-
- Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. J Am Coll Cardiol 2016671235–1250doi: 10.1016/j.jacc.2015.10.005 - PubMed
-
- Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, et al. ; ESC Scientific Document Group 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 20194087–165doi: 10.1093/eurheartj/ehy394 - PubMed
-
- Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Jüni P, Kastrati A, Kolh P, Mauri L, et al. ; ESC Scientific Document Group; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 201839213–260doi: 10.1093/eurheartj/ehx419 - PubMed
-
- Alexopoulos D, Xanthopoulou I, Gkizas V, Kassimis G, Theodoropoulos KC, Makris G, Koutsogiannis N, Damelou A, Tsigkas G, Davlouros P, et al. Randomized assessment of ticagrelor versus prasugrel antiplatelet effects in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv 20125797–804doi: 10.1161/CIRCINTERVENTIONS.112.972323 - PubMed

