SARS-CoV-2 Testing in Patients With Cancer Treated at a Tertiary Care Hospital During the COVID-19 Pandemic
- PMID: 32795227
- PMCID: PMC7571795
- DOI: 10.1200/JCO.20.01442
SARS-CoV-2 Testing in Patients With Cancer Treated at a Tertiary Care Hospital During the COVID-19 Pandemic
Abstract
Purpose: To analyze the prevalence of SARS-CoV-2 infection in patients with cancer in hospital care after implementation of institutional and governmental safety measurements.
Methods: Patients with cancer routinely tested for SARS-CoV-2 RNA by nasal swab and real-time polymerase chain reaction between March 21 and May 4, 2020, were included. The results of this cancer cohort were statistically compared with the SARS-CoV-2 prevalence in the Austrian population as determined by a representative nationwide random sample study (control cohort 1) and a cohort of patients without cancer presenting to our hospital (control cohort 2).
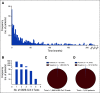
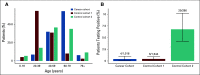
Results: A total of 1,688 SARS-CoV-2 tests in 1,016 consecutive patients with cancer were performed. A total of 270 of 1,016 (26.6%) of the patients were undergoing active anticancer treatment in a neoadjuvant/adjuvant and 560 of 1,016 (55.1%) in a palliative setting. A total of 53 of 1,016 (5.2%) patients self-reported symptoms potentially associated with COVID-19. In 4 of 1,016 (0.4%) patients, SARS-CoV-2 was detected. At the time of testing at our department, all four SARS-CoV-2-positive patients were asymptomatic, and two of them had recovered from symptomatic COVID-19. Viral clearance was achieved in three of the four patients 14-56 days after testing positive. The estimated odds ratio of SARS-CoV-2 prevalence between the cancer cohort and control cohort 1 was 1.013 (95% CI, 0.209 to 4.272; P = 1), and between control cohort 2 and the cancer cohort it was 18.333 (95% CI, 6.056 to 74.157).
Conclusion: Our data indicate that continuation of active anticancer therapy and follow-up visits in a large tertiary care hospital are feasible and safe after implementation of strict population-wide and institutional safety measures during the current COVID-19 pandemic. Routine SARS-CoV-2 testing of patients with cancer seems advisable to detect asymptomatic virus carriers and avoid uncontrolled viral spread.
Figures


References
-
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239. - PubMed
-
- World Health Organization: WHO announces COVID-19 outbreak a pandemic. http://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-....
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

