Seeing (and Using) the Light: Recent Developments in Bioluminescence Technology
- PMID: 32795417
- PMCID: PMC7472846
- DOI: 10.1016/j.chembiol.2020.07.022
Seeing (and Using) the Light: Recent Developments in Bioluminescence Technology
Abstract
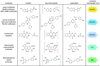
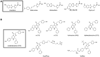
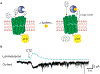
Bioluminescence has long been used to image biological processes in vivo. This technology features luciferase enzymes and luciferin small molecules that produce visible light. Bioluminescent photons can be detected in tissues and live organisms, enabling sensitive and noninvasive readouts on physiological function. Traditional applications have focused on tracking cells and gene expression patterns, but new probes are pushing the frontiers of what can be visualized. The past few years have also seen the merger of bioluminescence with optogenetic platforms. Luciferase-luciferin reactions can drive light-activatable proteins, ultimately triggering signal transduction and other downstream events. This review highlights these and other recent advances in bioluminescence technology, with an emphasis on tool development. We showcase how new luciferins and engineered luciferases are expanding the scope of optical imaging. We also highlight how bioluminescent systems are being leveraged not just for sensing-but also controlling-biological processes.
Keywords: bioluminescence; imaging; luciferase; luciferin; optogenetics.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures

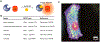


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

