Text Messaging Adherence Intervention for Adolescents and Young Adults with Chronic Kidney Disease: Pilot Randomized Controlled Trial and Stakeholder Interviews
- PMID: 32795983
- PMCID: PMC7455868
- DOI: 10.2196/19861
Text Messaging Adherence Intervention for Adolescents and Young Adults with Chronic Kidney Disease: Pilot Randomized Controlled Trial and Stakeholder Interviews
Abstract
Background: Up to one-third of adolescents and young adults (11-21 years old) with chronic kidney disease exhibit suboptimal rates of adherence to renal-protective antihypertensive medications. Mobile health interventions may promote higher adherence to these medicines in these individuals, but empirical research is needed to inform best practices for applying these modalities.
Objective: In this multiphase investigation, we developed and tested a theoretically informed text messaging intervention based on the COM-B model, a well-established health intervention framework stating that capability, opportunity, and motivation interactively modify health behaviors, to improve participants' antihypertensive medication adherence in a pilot randomized controlled trial. Qualitative data on user experiences were obtained.
Methods: In phase 1, intervention messages (Reminder+COM-B Message) were developed via stakeholder engagement of participants and pediatric nephrologists. In phase 2, the Reminder+COM-B Message intervention was tested against a Reminder-only Message active control condition in an 8-week pilot randomized controlled trial. The primary outcome was daily electronically monitored antihypertensive medication adherence and secondary outcomes included pre-post participant surveys of adherence self-efficacy, adherence barriers, outcome expectancies for taking medicine, and motivation for and importance of taking medicine. In phase 3, qualitative interviews related to user experiences were conducted with participants in the Reminder+COM-B Message intervention group.
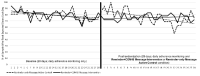
Results: Following phase 1, 34 participants (mean age 16.59 years, 41% female, 38% African American/Black, 35% hypertension diagnosis) completed the phase 2 pilot randomized controlled trial (n=18 in the Reminder+COM-B Message intervention group, n=16 in the Reminder-only Message active control group). All participants in the Reminder+COM-B Message intervention group completed a phase 3 qualitative interview. Overall, study procedures were feasible and the Reminder+COM-B Message intervention was acceptable to the participants (eg, 15/18 participants reported reading the majority of messages sent to them, 0/18 reported that the messages reduced their desire to take medicine). Prerandomization, there were no significant group differences in the rate of change in daily adherence over time. However, postrandomization, there was a significant group by time interaction (B=.01, P=.04) in which daily adherence decreased significantly over time in the Reminder-only Message active control group but remained stable in the Reminder+COM-B Message intervention group. There were no significant differences between groups in pre-post changes in survey responses. Qualitative interviews revealed participants' perceptions of how the Reminder+COM-B Message intervention changed adherence behavior and highlighted several areas for improving the intervention (eg, adapt messaging timing, intensity, and content to match daily adherence, send praise when medicine is taken).
Conclusions: The Reminder+COM-B Message intervention was feasible and acceptable to adolescents/young adults and demonstrated potential to promote participants' daily medication adherence beyond simple reminders. Further research is needed to determine the Reminder+COM-B Message intervention's mechanisms of adherence behavior change and to incorporate qualitative participant feedback into a modified version of this intervention to enhance its acceptability.
Trial registration: ClinicalTrials.gov NCT03651596; https://clinicaltrials.gov/ct2/show/NCT03651596.
Keywords: adherence; adolescent; intervention; kidney; kidney diseases; mHealth; medication adherence; mobile health; pediatrics; young adult.
©Cyd Eaton, Margaret Comer, Cozumel Pruette, Kevin Psoter, Kristin Riekert. Originally published in the Journal of Medical Internet Research (http://www.jmir.org), 14.08.2020.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

References
-
- Wühl E, Schaefer F. Therapeutic strategies to slow chronic kidney disease progression. Pediatr Nephrol. 2008 May;23(5):705–716. doi: 10.1007/s00467-008-0789-y. http://europepmc.org/abstract/MED/18335252 - DOI - PMC - PubMed
-
- Pruette CS, Coburn SS, Eaton CK, Brady TM, Tuchman S, Mendley S, Fivush BA, Eakin MN, Riekert KA. Does a multimethod approach improve identification of medication nonadherence in adolescents with chronic kidney disease? Pediatr Nephrol. 2019 Jan;34(1):97–105. doi: 10.1007/s00467-018-4044-x. http://europepmc.org/abstract/MED/30116892 - DOI - PMC - PubMed
-
- Hommel K, Ramsey R, Rich K, Ryan J. Adherence to pediatric treatment regimens. In: Roberts MC, Steele RG, editors. Handbook of pediatric psychology, 5th edition. New York: Guilford Press; 2017. pp. 119–133.
-
- Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, Agoritsas T, Mistry N, Iorio A, Jack S, Sivaramalingam B, Iserman E, Mustafa RA, Jedraszewski D, Cotoi C, Haynes RB. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014 Nov 20;(11):CD000011. doi: 10.1002/14651858.CD000011.pub4. http://europepmc.org/abstract/MED/25412402 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

