Sex- and cell-dependent contribution of peripheral high mobility group box 1 and TLR4 in arthritis-induced pain
- PMID: 32796317
- PMCID: PMC7808351
- DOI: 10.1097/j.pain.0000000000002034
Sex- and cell-dependent contribution of peripheral high mobility group box 1 and TLR4 in arthritis-induced pain
Abstract
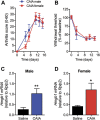
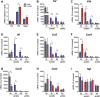
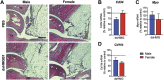
Spinal high mobility group box 1 protein (HMGB1) plays crucial roles in arthritis-induced pain; however, the involvement of peripheral HMGB1 has not been examined previously. In this study, we addressed the role of peripheral HMGB1 and explored if sex contributes differentially to nociception in arthritis. We found Hmgb1 expression to be elevated in the ankle joints of male and female mice subjected to collagen antibody-induced arthritis. Blocking the action of peripheral HMGB1, however, only reversed collagen antibody-induced arthritis-mediated hypersensitivity in males. Intra-articular injection of the toll-like receptor (TLR)4-activating, partially reduced disulfide, but not the fully reduced all-thiol, HMGB1 evoked mechanical hypersensitivity in both sexes. A sex-dependent temporal profile in expression of inflammatory factors in the ankle joint was observed in response to intra-articular injection of disulfide HMGB1, with male mice showing a delayed, yet longer-lasting increase in mRNA levels for several of the investigated factors. Intra-articular HMGB1 did not induce cellular infiltration in the ankle joint suggesting its action on tissue resident cells. To further explore possible sex differences in cellular involvement, we used the macrophage inhibitor, minocycline, and mice with specific TLR4 depletion in myeloid cells or nociceptors. We found that inhibition of resident macrophages attenuated HMGB1-induced pain-like behavior only in male mice. Interestingly, although the contribution of TLR4 on myeloid cells to nociception was minimal in females compared to males, TLR4 on nociceptors are important for HMGB1-induced pain in both sexes. Collectively, our work highlights sex- and cellular location-dependent roles of HMGB1 and TLR4 in peripheral pain mechanisms.
Copyright © 2020 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the International Association for the Study of Pain.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures





Similar articles
-
Spinal HMGB1 induces TLR4-mediated long-lasting hypersensitivity and glial activation and regulates pain-like behavior in experimental arthritis.Pain. 2014 Sep;155(9):1802-1813. doi: 10.1016/j.pain.2014.06.007. Epub 2014 Jun 20. Pain. 2014. PMID: 24954167
-
Sex-dependent role of microglia in disulfide high mobility group box 1 protein-mediated mechanical hypersensitivity.Pain. 2021 Feb 1;162(2):446-458. doi: 10.1097/j.pain.0000000000002033. Pain. 2021. PMID: 32773600 Free PMC article.
-
Spinal high-mobility group box-1 induces long-lasting mechanical hypersensitivity through the toll-like receptor 4 and upregulation of interleukin-1β in activated astrocytes.J Neurochem. 2019 Sep;150(6):738-758. doi: 10.1111/jnc.14812. Epub 2019 Jul 28. J Neurochem. 2019. PMID: 31273787
-
Pain in rheumatoid arthritis: Emerging role of high mobility group box 1 protein-HMGB1.Life Sci. 2025 Feb 1;362:123361. doi: 10.1016/j.lfs.2024.123361. Epub 2025 Jan 4. Life Sci. 2025. PMID: 39761742 Review.
-
[The Immunosuppressive Function of Myeloid-derived Suppressor Cells Is Regulated by the HMGB1-TLR4 Axis].Yakugaku Zasshi. 2018;138(2):143-148. doi: 10.1248/yakushi.17-00158. Yakugaku Zasshi. 2018. PMID: 29386427 Review. Japanese.
Cited by
-
The Neuroimmunology of Chronic Pain: From Rodents to Humans.J Neurosci. 2021 Feb 3;41(5):855-865. doi: 10.1523/JNEUROSCI.1650-20.2020. Epub 2020 Nov 25. J Neurosci. 2021. PMID: 33239404 Free PMC article. Review.
-
Innate Immunity and Sex: Distinct Inflammatory Profiles Associated with Murine Pain in Acute Synovitis.Cells. 2023 Jul 22;12(14):1913. doi: 10.3390/cells12141913. Cells. 2023. PMID: 37508577 Free PMC article.
-
Innate Immunity at the Core of Sex Differences in Osteoarthritic Pain?Front Pharmacol. 2022 May 19;13:881500. doi: 10.3389/fphar.2022.881500. eCollection 2022. Front Pharmacol. 2022. PMID: 35662714 Free PMC article. Review.
-
Macrophage as a Peripheral Pain Regulator.Cells. 2021 Jul 24;10(8):1881. doi: 10.3390/cells10081881. Cells. 2021. PMID: 34440650 Free PMC article. Review.
-
Protease-Activated Receptor 2 (PAR2) Expressed in Sensory Neurons Contributes to Signs of Pain and Neuropathy in Paclitaxel Treated Mice.J Pain. 2023 Nov;24(11):1980-1993. doi: 10.1016/j.jpain.2023.06.006. Epub 2023 Jun 12. J Pain. 2023. PMID: 37315729 Free PMC article.
References
-
- Agalave NM, Larsson M, Abdelmoaty S, Su J, Baharpoor A, Lundback P, Palmblad K, Andersson U, Harris H, Svensson CI. Spinal HMGB1 induces TLR4-mediated long-lasting hypersensitivity and glial activation and regulates pain-like behavior in experimental arthritis. PAIN 2014;155:1802–13. - PubMed
-
- Bas DB, Su J, Sandor K, Agalave NM, Lundberg J, Codeluppi S, Baharpoor A, Nandakumar KS, Holmdahl R, Svensson CI. Collagen antibody-induced arthritis evokes persistent pain with spinal glial involvement and transient prostaglandin dependency. Arthritis Rheum 2012;64:3886–96. - PubMed
-
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53:55–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

