Glucose Biosensor Based on Disposable Activated Carbon Electrodes Modified with Platinum Nanoparticles Electrodeposited on Poly(Azure A)
- PMID: 32796638
- PMCID: PMC7472169
- DOI: 10.3390/s20164489
Glucose Biosensor Based on Disposable Activated Carbon Electrodes Modified with Platinum Nanoparticles Electrodeposited on Poly(Azure A)
Abstract
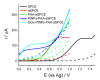
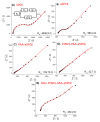
Herein, a novel electrochemical glucose biosensor based on glucose oxidase (GOx) immobilized on a surface containing platinum nanoparticles (PtNPs) electrodeposited on poly(Azure A) (PAA) previously electropolymerized on activated screen-printed carbon electrodes (GOx-PtNPs-PAA-aSPCEs) is reported. The resulting electrochemical biosensor was validated towards glucose oxidation in real samples and further electrochemical measurement associated with the generated H2O2. The electrochemical biosensor showed an excellent sensitivity (42.7 μA mM-1 cm-2), limit of detection (7.6 μM), linear range (20 μM-2.3 mM), and good selectivity towards glucose determination. Furthermore, and most importantly, the detection of glucose was performed at a low potential (0.2 V vs. Ag). The high performance of the electrochemical biosensor was explained through surface exploration using field emission SEM, XPS, and impedance measurements. The electrochemical biosensor was successfully applied to glucose quantification in several real samples (commercial juices and a plant cell culture medium), exhibiting a high accuracy when compared with a classical spectrophotometric method. This electrochemical biosensor can be easily prepared and opens up a good alternative in the development of new sensitive glucose sensors.
Keywords: activated screen-printed carbon electrodes; enzymatic biosensor; glucose; glucose oxidase; platinum nanoparticles; poly(Azure A).
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Samphao A., Butmee P., Jitcharoen J., Svorc L.U., Raber G., Kalcher K. Flow-injection amperometric determination of glucose using a biosensor based on immobilization of glucose oxidase onto Au seeds decorated on core Fe3O4 nanoparticles. Talanta. 2015;142:35–42. doi: 10.1016/j.talanta.2015.01.046. - DOI - PubMed
-
- Wilson A.M., Work T.M., Bushway A.A., Bushway R.J. HPLC determination of fructose, glucose, and sucrose in potatoes. J. Food Sci. 1981;46:300–301. doi: 10.1111/j.1365-2621.1981.tb14589.x. - DOI
-
- Wang X.Y., Chen Y., Li Z., Wang Z. Analysis of carbohydrates by capillary zone electrophoresis with on-capillary derivatization. J. Liq. Chromatogr. Related Technol. 2002;25:589–600. doi: 10.1081/JLC-120008813. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

