Like a Rolling Stone: Sting-Cgas Pathway and Cell-Free DNA as Biomarkers for Combinatorial Immunotherapy
- PMID: 32796670
- PMCID: PMC7464249
- DOI: 10.3390/pharmaceutics12080758
Like a Rolling Stone: Sting-Cgas Pathway and Cell-Free DNA as Biomarkers for Combinatorial Immunotherapy
Abstract
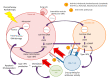
Combining immune checkpoint inhibitors with other treatments likely to harness tumor immunity is a rising strategy in oncology. The exact modalities of such a combinatorial regimen are yet to be defined, and most attempts have relied so far on concomitant dosing, rather than sequential or phased administration. Because immunomodulating features are likely to be time-, dose-, and-schedule dependent, the need for biomarkers providing real-time information is critical to better define the optimal time-window to combine immune checkpoint inhibitors with other drugs. In this review, we present the various putative markers that have been investigated as predictive tools with immune checkpoint inhibitors and could be used to help further combining treatments. Whereas none of the current biomarkers, such as the PDL1 expression of a tumor mutational burden, is suitable to identify the best way to combine treatments, monitoring circulating tumor DNA is a promising strategy, in particular to check whether the STING-cGAS pathway has been activated by cytotoxics. As such, circulating tumor DNA could help defining the best time-window to administrate immune checkpoint inhibitors after that cytotoxics have been given.
Keywords: biomarkers; combinatorial immunotherapy; cytotoxics; precision medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Barlési F., Scherpereel A., Rittmeyer A., Pazzola A., Tur N.F., Kim J.-H., Ahn M.-J., Aerts J.G., Gorbunova V., Vikström A., et al. Randomized Phase III Trial of Maintenance Bevacizumab with or without Pemetrexed after First-Line Induction with Bevacizumab, Cisplatin, and Pemetrexed in Advanced Nonsquamous Non–Small-Cell Lung Cancer: AVAPERL (MO22089) J. Clin. Oncol. 2013;31:3004–3011. doi: 10.1200/JCO.2012.42.3749. - DOI - PubMed
-
- Kaufman H.L., Atkins M.B., Subedi P., Wu J., Chambers J., Mattingly T.J., Campbell J.D., Allen J., Ferris A.E., Schilsky R.L., et al. The promise of Immuno-oncology: Implications for defining the value of cancer treatment. J. Immunother. Cancer. 2019;7:129. doi: 10.1186/s40425-019-0594-0. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

