Vascular Remodeling in Moyamoya Angiopathy: From Peripheral Blood Mononuclear Cells to Endothelial Cells
- PMID: 32796702
- PMCID: PMC7460840
- DOI: 10.3390/ijms21165763
Vascular Remodeling in Moyamoya Angiopathy: From Peripheral Blood Mononuclear Cells to Endothelial Cells
Abstract
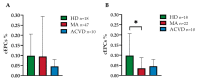
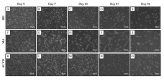
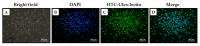
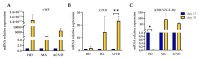
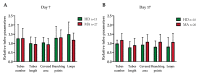
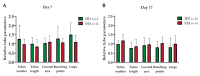
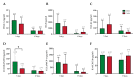
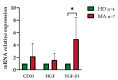
The pathophysiological mechanisms of Moyamoya angiopathy (MA), which is a rare cerebrovascular condition characterized by recurrent ischemic/hemorrhagic strokes, are still largely unknown. An imbalance of vasculogenic/angiogenic mechanisms has been proposed as one possible disease aspect. Circulating endothelial progenitor cells (cEPCs) have been hypothesized to contribute to vascular remodeling of MA, but it remains unclear whether they might be considered a disease effect or have a role in disease pathogenesis. The aim of the present study was to provide a morphological, phenotypical, and functional characterization of the cEPCs from MA patients to uncover their role in the disease pathophysiology. cEPCs were identified from whole blood as CD45dimCD34+CD133+ mononuclear cells. Morphological, biochemical, and functional assays were performed to characterize cEPCs. A significant reduced level of cEPCs was found in blood samples collected from a homogeneous group of adult (mean age 46.86 ± 11.7; 86.36% females), Caucasian, non-operated MA patients with respect to healthy donors (HD; p = 0.032). Since no difference in cEPC characteristics and functionality was observed between MA patients and HD, a defective recruitment mechanism could be involved in the disease pathophysiology. Collectively, our results suggest that cEPC level more than endothelial progenitor cell (EPC) functionality seems to be a potential marker of MA. The validation of our results on a larger population and the correlation with clinical data as well as the use of more complex cellular model could help our understanding of EPC role in MA pathophysiology.
Keywords: Moyamoya angiopathy; RNF213; endothelial progenitor cells; neovascularization.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Fukui M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis (’moyamoya’ disease). Research committee on spontaneous occlusion of the circle of willis (moyamoya disease) of the ministry of health and welfare, Japan. Clin. Neurol. Neurosurg. 1997;99:S238–S240. doi: 10.1016/S0303-8467(97)00082-6. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

