Ischemia induces autophagy of endothelial cells and stimulates angiogenic effects in a hindlimb ischemia mouse model
- PMID: 32796816
- PMCID: PMC7429831
- DOI: 10.1038/s41419-020-02849-4
Ischemia induces autophagy of endothelial cells and stimulates angiogenic effects in a hindlimb ischemia mouse model
Abstract
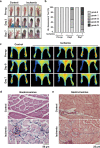
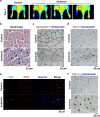
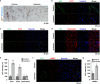
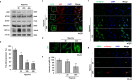
Although peripheral artery disease (PAD) is a major health problem, there have been limited advances in medical therapies. In PAD patients, angiogenesis is regarded as a promising therapeutic strategy to promote new arterial vessels and improve perfusion of ischemic tissue. Autophagy plays a critical role in catabolic processes for cell survival under normal and stressful conditions and plays fundamental biological roles in various cellular functions. In the present study, we showed that autophagy in endothelial cells is important for the repair and regeneration of damaged tissues. In a hindlimb ischemia mouse model, autophagy was stimulated in endothelial cells of the quadriceps muscle, and adjacent cells proliferated and regenerated. The autophagy pathway was induced under prolonged hypoxia in endothelial cells, and autophagy increased angiogenic activities. Moreover, conditioned media from endothelial cells blocked autophagy and inhibited the proliferation of muscle cells, suggesting that autophagic stimulation in endothelial cells affects the survival of adjacent cells, such as muscle. Collectively, hypoxia/ischemia-induced autophagy angiogenesis, and the damaged tissue surrounded by neo-vessels was regenerated in an ischemia model. Therefore, we strongly suggest that stimulation of autophagy in endothelial cells may be a potent therapeutic strategy in severe vascular diseases, including PAD.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

