Distinct fibroblast subsets regulate lacteal integrity through YAP/TAZ-induced VEGF-C in intestinal villi
- PMID: 32796823
- PMCID: PMC7428020
- DOI: 10.1038/s41467-020-17886-y
Distinct fibroblast subsets regulate lacteal integrity through YAP/TAZ-induced VEGF-C in intestinal villi
Abstract
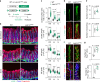
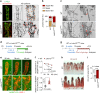
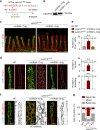
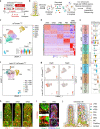
Emerging evidence suggests that intestinal stromal cells (IntSCs) play essential roles in maintaining intestinal homeostasis. However, the extent of heterogeneity within the villi stromal compartment and how IntSCs regulate the structure and function of specialized intestinal lymphatic capillary called lacteal remain elusive. Here we show that selective hyperactivation or depletion of YAP/TAZ in PDGFRβ+ IntSCs leads to lacteal sprouting or regression with junctional disintegration and impaired dietary fat uptake. Indeed, mechanical or osmotic stress regulates IntSC secretion of VEGF-C mediated by YAP/TAZ. Single-cell RNA sequencing delineated novel subtypes of villi fibroblasts that upregulate Vegfc upon YAP/TAZ activation. These populations of fibroblasts were distributed in proximity to lacteal, suggesting that they constitute a peri-lacteal microenvironment. Our findings demonstrate the heterogeneity of IntSCs and reveal that distinct subsets of villi fibroblasts regulate lacteal integrity through YAP/TAZ-induced VEGF-C secretion, providing new insights into the dynamic regulatory mechanisms behind lymphangiogenesis and lymphatic remodeling.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Bernier-Latmani J, Petrova TV. Intestinal lymphatic vasculature: structure, mechanisms and functions. Nature reviews. Gastroenterol. Hepatol. 2017;14:510–526. - PubMed
-
- Aspelund A, Robciuc MR, Karaman S, Makinen T, Alitalo K. Lymphatic system in cardiovascular medicine. Circ. Res. 2016;118:515–530. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

