High seroprevalence for SARS-CoV-2 among household members of essential workers detected using a dried blood spot assay
- PMID: 32797108
- PMCID: PMC7428174
- DOI: 10.1371/journal.pone.0237833
High seroprevalence for SARS-CoV-2 among household members of essential workers detected using a dried blood spot assay
Abstract
Objective: Serological testing is needed to investigate the extent of transmission of SARS-CoV-2 from front-line essential workers to their household members. However, the requirement for serum/plasma limits serological testing to clinical settings where it is feasible to collect and process venous blood. To address this problem we developed a serological test for SARS-CoV-2 IgG antibodies that requires only a single drop of finger stick capillary whole blood, collected in the home and dried on filter paper (dried blood spot, DBS). We describe assay performance and demonstrate its utility for remote sampling with results from a community-based study.
Methods: An ELISA to the receptor binding domain of the SARS-CoV-2 spike protein was optimized to quantify IgG antibodies in DBS. Samples were self-collected from a community sample of 232 participants enriched with health care workers, including 30 known COVID-19 cases and their household members.
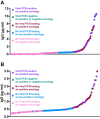
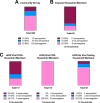
Results: Among 30 individuals sharing a household with a virus-confirmed case of COVID-19, 80% were seropositive. Of 202 community individuals without prior confirmed acute COVID-19 diagnoses, 36% were seropositive. Of documented convalescent COVID-19 cases from the community, 29 of 30 (97%) were seropositive for IgG antibodies to the receptor binding domain.
Conclusion: DBS ELISA provides a minimally-invasive alternative to venous blood collection. Early analysis suggests a high rate of transmission among household members. High rates of seroconversion were also noted following recovery from infection. Serological testing for SARS-CoV-2 IgG antibodies in DBS samples can facilitate seroprevalence assessment in community settings to address epidemiological questions, monitor duration of antibody responses, and assess if antibodies against the spike protein correlate with protection from reinfection.
Conflict of interest statement
The authors of this paper have read the journal's policy and have the following competing interests: Thomas W. McDade has a financial interest in EnMed Microanalytics, a company that performs laboratory tests using dried blood spot samples. There are no patents, products in development or marketed products associated with this research to declare. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


References
-
- Centers for Disease Control and Prevention. Shipping guidelines for dried blood spot specimens. 2017: https://www.cdc.gov/labstandards/pdf/nsqap/Bloodspot_Transportation_Guid....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

