Development and evaluation of a Novel RT-PCR system for reliable and rapid SARS-CoV-2 screening of blood donations
- PMID: 32798248
- PMCID: PMC7461364
- DOI: 10.1111/trf.16049
Development and evaluation of a Novel RT-PCR system for reliable and rapid SARS-CoV-2 screening of blood donations
Abstract
Background: The ongoing outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused great global concerns. In contrast to SARS, some SARS-CoV-2-infected people can be asymptomatic or have only mild nonspecific symptoms. Furthermore, there is evidence that SARS-CoV-2 may be infectious during an asymptomatic incubation period. With the discovery that SARS-CoV-2 can be detected in plasma or serum, blood safety is worthy of consideration.
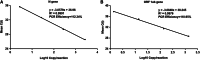
Study design and methods: We developed a nucleic acid test (NAT) screening system for SARS-CoV-2 targeting nucleocapsid protein (N) and open reading frame 1ab (ORF 1ab) gene that could screen 5076 samples every 24 hours. The 2019 novel coronavirus RNA standard was used to evaluate linearity of standard curves. Diagnostic sensitivity and reproducibility were evaluated using artificial SARS-CoV-2. Specificity was evaluated with 61 other respiratory pathogens. Diagnostic performance was evaluated by testing two sputum and nine oropharyngeal swab specimens. The reverse transcription polymerase chain reaction (RT-PCR) assay was used to screen SARS-CoV-2 RNA in blood donor specimens collected during the outbreak of SARS-CoV-2 in Chengdu.
Results: Limits of detection of the SARS-CoV-2 RT-PCR assay for N and ORF 1ab gene were 12.5 and 27.58 copies/mL, respectively. Intra-assay and interassay for the SARS-CoV-2 RT-PCR assay based on cycle threshold were acceptably low. No cross-reactivity was observed with other respiratory virus and bacterial isolates. The overall agreement value between the SARS-CoV-2 RT-PCR assay and clinical diagnostic results was 100%. A total of 16 287 blood specimens collected from blood donors during SARS-CoV-2 surveillance were tested negative.
Conclusions: A high-throughput NAT screening system was developed for SARS-CoV-2 screening of blood donations during the outbreak of SARS-CoV-2.
© 2020 AABB.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

