Improvement of Severe Fatigue Following Nuclease Therapy in Patients With Primary Sjögren's Syndrome: A Randomized Clinical Trial
- PMID: 32798283
- PMCID: PMC7839752
- DOI: 10.1002/art.41489
Improvement of Severe Fatigue Following Nuclease Therapy in Patients With Primary Sjögren's Syndrome: A Randomized Clinical Trial
Abstract
Objective: To assess the safety and efficacy of RSLV-132, an RNase Fc fusion protein, in a phase II randomized, double-blind, placebo-controlled clinical trial in patients with primary Sjögren's syndrome (SS).
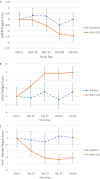
Methods: Thirty patients with primary SS were randomized to receive treatment with RSLV-132 or placebo intravenously once per week for 2 weeks, and then every 2 weeks for 12 weeks. Eight patients received placebo and 20 patients received RSLV-132 at a dose of 10 mg/kg. Clinical efficacy measures included the European League Against Rheumatism (EULAR) Sjögren's Syndrome Disease Activity Index, EULAR Sjögren's Syndrome Patient Reported Index (ESSPRI), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Profile of Fatigue (ProF), and the Digit Symbol Substitution Test (DSST).
Results: Patients randomized to receive RSLV-132 experienced clinically meaningful improvements in the ESSPRI score (P = 0.27), FACIT-F score (P = 0.05), ProF score (P = 0.07), and DSST (P = 0.02) from baseline to day 99, whereas patients who received placebo showed no changes in any of these clinical efficacy measures. This improvement was significantly correlated with increased expression of selected interferon-inducible genes (Pearson's correlations, each P < 0.05).
Conclusion: Administration of RSLV-132 improved severe fatigue, as determined by 4 independent patient-reported measures of fatigue, in patients with primary SS.
Trial registration: ClinicalTrials.gov NCT03247686.
© 2020 Resolve Therapeutics, LLC. Arthritis & Rheumatology published by Wiley Periodicals, Inc. on behalf of American College of Rheumatology.
Figures


Comment in
-
Reply.Arthritis Rheumatol. 2021 Apr;73(4):718-719. doi: 10.1002/art.41581. Epub 2021 Feb 18. Arthritis Rheumatol. 2021. PMID: 33164338 No abstract available.
-
Should the Biopsychosocial Model Be Considered in Systemic Autoimmune Diseases? Comment on the Article by Posada et al.Arthritis Rheumatol. 2021 Apr;73(4):717-718. doi: 10.1002/art.41582. Epub 2021 Feb 18. Arthritis Rheumatol. 2021. PMID: 33164342 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

