H2S- and NO-releasing gasotransmitter platform: A crosstalk signaling pathway in the treatment of acute kidney injury
- PMID: 32798649
- PMCID: PMC7426260
- DOI: 10.1016/j.phrs.2020.105121
H2S- and NO-releasing gasotransmitter platform: A crosstalk signaling pathway in the treatment of acute kidney injury
Abstract
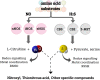
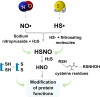
Acute kidney injury (AKI) is a syndrome affecting most patients hospitalized due to kidney disease; it accounts for 15 % of patients hospitalized in intensive care units worldwide. AKI is mainly caused by ischemia and reperfusion (IR) injury, which temporarily obstructs the blood flow, increases inflammation processes and induces oxidative stress. AKI treatments available nowadays present notable disadvantages, mostly for patients with other comorbidities. Thus, it is important to investigate different approaches to help minimizing side effects such as the ones observed in patients subjected to the aforementioned treatments. Therefore, the aim of the current review is to highlight the potential of two endogenous gasotransmitters - hydrogen sulfide (H2S) and nitric oxide (NO) - and their crosstalk in AKI treatment. Both H2S and NO are endogenous signalling molecules involved in several physiological and pathophysiological processes, such as the ones taking place in the renal system. Overall, these molecules act by decreasing inflammation, controlling reactive oxygen species (ROS) concentrations, activating/inactivating pro-inflammatory cytokines, as well as promoting vasodilation and decreasing apoptosis, hypertrophy and autophagy. Since these gasotransmitters are found in gaseous state at environmental conditions, they can be directly applied by inhalation, or in combination with H2S and NO donors, which are compounds capable of releasing these molecules at biological conditions, thus enabling higher stability and slow release of NO and H2S. Moreover, the combination between these donor compounds and nanomaterials has the potential to enable targeted treatments, reduce side effects and increase the potential of H2S and NO. Finally, it is essential highlighting challenges to, and perspectives in, pharmacological applications of H2S and NO to treat AKI, mainly in combination with nanoparticulated delivery platforms.
Keywords: Acute kidney injury; Hydrogen sulfide; Nanomaterials; Nitric oxide.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Mercado M.G., Smith D.K., Guard E.L. Acute kidney injury: diagnosis and management. Am. Fam. Physician. 2019;100:687–694. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

