TBCRC 030: a phase II study of preoperative cisplatin versus paclitaxel in triple-negative breast cancer: evaluating the homologous recombination deficiency (HRD) biomarker
- PMID: 32798689
- PMCID: PMC8437015
- DOI: 10.1016/j.annonc.2020.08.2064
TBCRC 030: a phase II study of preoperative cisplatin versus paclitaxel in triple-negative breast cancer: evaluating the homologous recombination deficiency (HRD) biomarker
Abstract
Background: Cisplatin and paclitaxel are active in triple-negative breast cancer (TNBC). Despite different mechanisms of action, effective predictive biomarkers to preferentially inform drug selection have not been identified. The homologous recombination deficiency (HRD) assay (Myriad Genetics, Inc.) detects impaired double-strand DNA break repair and may identify patients with BRCA1/2-proficient tumors that are sensitive to DNA-targeting therapy. The primary objective of TBCRC 030 was to detect an association of HRD with pathologic response [residual cancer burden (RCB)-0/1] to single-agent cisplatin or paclitaxel.
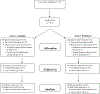
Patients and methods: This prospective phase II study enrolled patients with germline BRCA1/2 wild-type/unknown stage I-III TNBC in a 12-week randomized study of preoperative cisplatin or paclitaxel. The HRD assay was carried out on baseline tissue; positive HRD was defined as a score ≥33. Crossover to an alternative chemotherapy was offered if there was inadequate response.
Results: One hundred and thirty-nine patients were evaluable for response, including 88 (63.3%) who had surgery at 12 weeks and 51 (36.7%) who crossed over to an alternative provider-selected preoperative chemotherapy regimen due to inadequate clinical response. HRD results were available for 104 tumors (74.8%) and 74 (71.1%) were HRD positive. The RCB-0/1 rate was 26.4% with cisplatin and 22.3% with paclitaxel. No significant association was observed between HRD score and RCB response to either cisplatin [odds ratio (OR) for RCB-0/1 if HRD positive 2.22 (95% CI: 0.39-23.68)] or paclitaxel [OR for RCB-0/1 if HRD positive 0.90 (95% CI: 0.19-4.95)]. There was no evidence of an interaction between HRD and pathologic response to chemotherapy.
Conclusions: In this prospective preoperative trial in TNBC, HRD was not predictive of pathologic response. Tumors were similarly responsive to preoperative paclitaxel or cisplatin chemotherapy.
Keywords: HRD; Triple-negative breast cancer (TNBC); cisplatin; neoadjuvant; paclitaxel; preoperative.
Copyright © 2020 European Society for Medical Oncology. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Disclosures The authors have declared no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

