Activated Protein Phosphatase 2A Disrupts Nutrient Sensing Balance Between Mechanistic Target of Rapamycin Complex 1 and Adenosine Monophosphate-Activated Protein Kinase, Causing Sarcopenia in Alcohol-Associated Liver Disease
- PMID: 32799332
- PMCID: PMC8847884
- DOI: 10.1002/hep.31524
Activated Protein Phosphatase 2A Disrupts Nutrient Sensing Balance Between Mechanistic Target of Rapamycin Complex 1 and Adenosine Monophosphate-Activated Protein Kinase, Causing Sarcopenia in Alcohol-Associated Liver Disease
Abstract
Background and aims: Despite the high clinical significance of sarcopenia in alcohol-associated cirrhosis, there are currently no effective therapies because the underlying mechanisms are poorly understood. We determined the mechanisms of ethanol-induced impaired phosphorylation of mechanistic target of rapamycin complex 1 (mTORC1) and adenosine monophosphate-activated protein kinase (AMPK) with consequent dysregulated skeletal muscle protein homeostasis (balance between protein synthesis and breakdown).
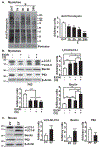
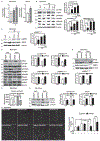
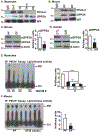
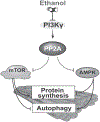
Approach and results: Differentiated murine myotubes, gastrocnemius muscle from mice with loss and gain of function of regulatory genes following ethanol treatment, and skeletal muscle from patients with alcohol-associated cirrhosis were used. Ethanol increases skeletal muscle autophagy by dephosphorylating mTORC1, circumventing the classical kinase regulation by protein kinase B (Akt). Concurrently and paradoxically, ethanol exposure results in dephosphorylation and inhibition of AMPK, an activator of autophagy and inhibitor of mTORC1 signaling. However, AMPK remains inactive with ethanol exposure despite lower cellular and tissue adenosine triphosphate, indicating a "pseudofed" state. We identified protein phosphatase (PP) 2A as a key mediator of ethanol-induced signaling and functional perturbations using loss and gain of function studies. Ethanol impairs binding of endogenous inhibitor of PP2A to PP2A, resulting in methylation and targeting of PP2A to cause dephosphorylation of mTORC1 and AMPK. Activity of phosphoinositide 3-kinase-γ (PI3Kγ), a negative regulator of PP2A, was decreased in response to ethanol. Ethanol-induced molecular and phenotypic perturbations in wild-type mice were observed in PI3Kγ-/- mice even at baseline. Importantly, overexpressing kinase-active PI3Kγ but not the kinase-dead mutant reversed ethanol-induced molecular perturbations.
Conclusions: Our study describes the mechanistic underpinnings for ethanol-mediated dysregulation of protein homeostasis by PP2A that leads to sarcopenia with a potential for therapeutic approaches by targeting the PI3Kγ-PP2A axis.
© 2020 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- R56 HL141744/HL/NHLBI NIH HHS/United States
- U01 DK061732/DK/NIDDK NIH HHS/United States
- R00 AA025386/AA/NIAAA NIH HHS/United States
- T32 DK083251/DK/NIDDK NIH HHS/United States
- R01 AA028190/AA/NIAAA NIH HHS/United States
- R21 AA022742/AA/NIAAA NIH HHS/United States
- U01 AA026976/AA/NIAAA NIH HHS/United States
- K08 AA028794/AA/NIAAA NIH HHS/United States
- L30 AA027927/AA/NIAAA NIH HHS/United States
- R01 DK113196/DK/NIDDK NIH HHS/United States
- P50 AA024333/AA/NIAAA NIH HHS/United States
- R01 GM119174/GM/NIGMS NIH HHS/United States
- R21 AR071046/AR/NIAMS NIH HHS/United States
- R01 HL089473/HL/NHLBI NIH HHS/United States
- R01 DK083414/DK/NIDDK NIH HHS/United States
- U01 AA021890/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

