Epilepsy in time of COVID-19: A survey-based study
- PMID: 32799337
- PMCID: PMC7460986
- DOI: 10.1111/ane.13335
Epilepsy in time of COVID-19: A survey-based study
Abstract
Objectives: Collateral damage may occur in epilepsy management during the coronavirus (COVID-19) pandemic. We aimed to establish the impact of this pandemic on epilepsy patients in terms of patient-reported seizure control and emerging symptoms.
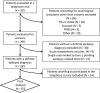
Materials & methods: This is a cross-sectional study including consecutive patients assessed by telephone contact in an epilepsy clinic during the first month of confinement. Demographic and clinical characteristics were recorded, and a 19-item questionnaire was systematically completed. Data regarding the impact of confinement, economic effects of the pandemic, and subjective perception of telemedicine were recorded. Additional clinical data were obtained in patients with a COVID-19 diagnosis.
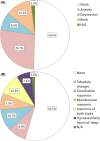
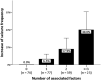
Results: Two hundred and fifty-five patients were recruited: mean age 48.2 ± 19.8 years, 121 (47.5%) women. An increase in seizure frequency was reported by 25 (9.8%) patients. Sixty-eight (26.7%) patients reported confinement-related anxiety, 22 (8.6%) depression, 31 (12.2%) both, and 72 (28.2%) insomnia. Seventy-three (28.6%) patients reported a reduction in economic income. Logistic regression analysis showed that tumor-related epilepsy etiology [OR = 7.36 (95% CI 2.17-24.96)], drug-resistant epilepsy [OR = 3.44 (95% CI 1.19-9.95)], insomnia [OR = 3.25 (95% CI 1.18-8.96)], fear of epilepsy [OR = 3.26 (95% CI 1.09-9.74)], and income reduction [OR = 3.65 (95% CI 1.21-10.95)] were associated with a higher risk of increased seizure frequency. Telemedicine was considered satisfactory by 214 (83.9%) patients. Five patients were diagnosed with COVID-19, with no changes in seizure frequency.
Conclusions: The COVID-19 pandemic has effects in epilepsy patients. Patients with tumor-related, drug-resistant epilepsy, insomnia, and economic difficulties are at a higher risk of increased seizure frequency. Telemedicine represents a suitable tool in this setting.
Keywords: coronavirus; epilepsy; pandemics; quality of life; seizures.
© 2020 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
E. Fonseca declares research funding and speaker fees from UCB Pharma, Esteve laboratorios, Eisai Inc, and Sanofi Genzyme. L. Abraira declares research funding and speaker fees from UCB Pharma, BIAL Pharmaceutical, EISAI Inc, Sanofi Genzyme, and Esteve laboratorios. E. Santamarina declares research funding and speaker fees from UCB Pharma, BIAL Pharmaceutical, EISAI Inc, Arvelle, and Esteve laboratorios. I. Seijo‐Raposo declares research funding from UCB Pharma, Neuraxpharm, and GW pharmaceuticals. M. Toledo declares research funding and speaker fees from UCB Pharma, BIAL Pharmaceutical, EISAI Inc, Sanofi, Arvelle, and Esteve laboratorios. M Quintana, S. Lallana, and JL. Restrepo have no conflicts of interest to declare.
Figures
Comment in
-
Epilepsy in the time of COVID-19.Acta Neurol Scand. 2021 Mar;143(3):333-335. doi: 10.1111/ane.13360. Epub 2020 Nov 6. Acta Neurol Scand. 2021. PMID: 33043445 Free PMC article. No abstract available.
References
-
- WHO‐China Joint Mission . Report of the WHO‐China Joint Mission on Coronavirus Disease 2019 (COVID‐19). https://reliefweb.int/sites/reliefweb.int/files/resources/who‐china‐join.... Accessed April 18, 2020
-
- Coronavirus (COVID‐19) events as they happen. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/events‐a.... Accessed April 18, 2020
-
- WHO COVID‐19 Dashboard. https://covid19.who.int/. Accessed April 22, 2020
-
- Situación de COVID‐19 o Coronavirus en España. https://covid19.isciii.es/. Accessed April 18, 2020

