Sulfur Metabolism Under Stress
- PMID: 32799544
- PMCID: PMC7699002
- DOI: 10.1089/ars.2020.8151
Sulfur Metabolism Under Stress
Abstract
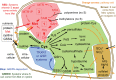
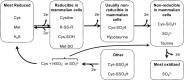
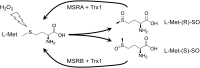
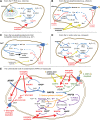
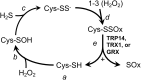
Significance: In humans, imbalances in the reduction-oxidation (redox) status of cells are associated with many pathological states. In addition, many therapeutics and prophylactics used as interventions for diverse pathologies either directly modulate oxidant levels or otherwise influence endogenous cellular redox systems. Recent Advances: The cellular machineries that maintain redox homeostasis or that function within antioxidant defense systems rely heavily on the regulated reactivities of sulfur atoms either within or derived from the amino acids cysteine and methionine. Recent advances have substantially advanced our understanding of the complex and essential chemistry of biological sulfur-containing molecules. Critical Issues: The redox machineries that maintain cellular homeostasis under diverse stresses can consume large amounts of energy to generate reducing power and/or large amounts of sulfur-containing nutrients to replenish or sustain intracellular stores. By understanding the metabolic pathways underlying these responses, one can better predict how to protect cells from specific stresses. Future Directions: Here, we summarize the current state of knowledge about the impacts of different stresses on cellular metabolism of sulfur-containing molecules. This analysis suggests that there remains more to be learned about how cells use sulfur chemistry to respond to stresses, which could in turn lead to advances in therapeutic interventions for some exposures or conditions.
Keywords: disulfide reductase systems; drug metabolism; methionine cycle; oxidative stress; trans-sulfuration.
Figures
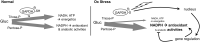
References
-
- Adams JD Jr., Lauterburg BH, and Mitchell JR.. Plasma glutathione and glutathione disulfide in the rat: regulation and response to oxidative stress. J Pharmacol Exp Ther 227: 749–754, 1983 - PubMed
-
- Akaike T, Ida T, Wei FY, Nishida M, Kumagai Y, Alam MM, Ihara H, Sawa T, Matsunaga T, Kasamatsu S, Nishimura A, Morita M, Tomizawa K, Nishimura A, Watanabe S, Inaba K, Shima H, Tanuma N, Jung M, Fujii S, Watanabe Y, Ohmuraya M, Nagy P, Feelisch M, Fukuto JM, and Motohashi H. Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat Commun 8: 1177, 2017 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

