Establishing a high sensitivity detection method for SARS-CoV-2 IgM/IgG and developing a clinical application of this method
- PMID: 32799618
- PMCID: PMC7534335
- DOI: 10.1080/22221751.2020.1811161
Establishing a high sensitivity detection method for SARS-CoV-2 IgM/IgG and developing a clinical application of this method
Abstract
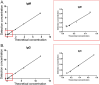
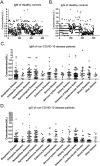
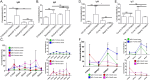
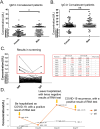
COVID-19 is caused by SARS-CoV-2 infection and was initially discovered in Wuhan. This outbreak quickly spread all over China and then to more than 20 other countries. SARS-CoV-2 fluorescent microsphere immunochromatographic test strips were prepared by the combination of time-resolved fluorescence immunoassay with a lateral flow assay. The analytical performance and clinical evaluation of this testing method was done and the clinical significance of the testing method was verified. The LLOD of SARS-CoV-2 antibody IgG and IgM was 0.121U/L and 0.366U/L. The specificity of IgM and IgG strips in healthy people and in patients with non-COVID-19 disease was 94%, 96.72% and 95.50%, 99.49%, respectively; and sensitivity of IgM and IgG strips for patients during treatment and follow-up was 63.02%, 37.61% and 87.28%, 90.17%, respectively. The SARS-CoV-2 antibody test strip can provide rapid, flexible and accurate testing, and is able to meet the clinical requirement for rapid on-site testing of virus. The ability to detect IgM and IgG provided a significant benefit for the detection and prediction of clinical course with COVID-19 patients.
Keywords: SARS-CoV-2 antibody; disease evaluation; multi-epitopes fusion protein; prognosis; time-resolved fluorescence immunoassay.
Conflict of interest statement
XXL and ZJP are employees of Beijing Diagreat Biotechnology, the commercial manufacturer of the SARS-CoV-2 IgM/IgG test strips.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
