BIP and the unfolded protein response are important for potyvirus and potexvirus infection
- PMID: 32799639
- PMCID: PMC7598082
- DOI: 10.1080/15592324.2020.1807723
BIP and the unfolded protein response are important for potyvirus and potexvirus infection
Abstract
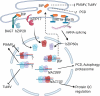
Plant potexvirus and potyvirus infection can trigger endoplasmic reticulum (ER) stress. ER stress signaling increases the expression of cytoprotective ER-chaperones, especially the BiP chaperones which contribute to pro-survival functions when plants are subjected to infection. The inositol requiring enzyme (IRE1) is one ER stress sensor that is activated to splice the bZIP60 mRNA which produces a truncated transcription factor that activates gene expression in the nucleus. The IRE1/bZIP60 pathway is associated with restricting potyvirus and potexvirus infection. Recent data also identified the IRE1-independent UPR pathways led by bZIP28 and bZIP17 contribute to potexvirus and potyvirus infection. These three bZIP pathways recognize cis-regulatory elements in the BiP promoters to enhance gene expression. BiP is part of a negative feedback loop that regulates the activities of the ER stress transducers IRE1, bZIP28, and bZIP17 to block their activation. We discuss a model in which bZIP60 and bZIP17 synergistically induce BiP and other genes restricting Plantago asiatica mosaic virus (PlAMV; a potexvirus) infection while bZIP60 and bZIP28 independently induce genes supporting PlAMV infection. Regarding Turnip mosiac virus (TuMV, a potyvirus) infection, bZIP60 and bZIP28 serve to repress local and systemic infection. Finally, tauroursodeoxycholic acid treatments were used to demonstrate that the protein folding capacity significantly influences PlAMV accumulation.
Keywords: BIP; Unfolded protein response; endoplasmic reticulum stress; molecular chaperones; plant stress; potexvirus; potyvirus.
Figures
References
-
- Leborgne-Castel N, Jelitto-Van Dooren EP, Crofts AJ, Denecke J.. Overexpression of BiP in tobacco alleviates endoplasmic reticulum stress. Plant Cell [Internet] 1999; 11:1–5. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=144191&tool=pm.... - PMC - PubMed
-
- Srivastava R, Deng Y, Shah S, Rao AG, Howell SH.. Binding protein is a master regulator of the endoplasmic reticulum stress sensor/transducer bZIP28 in arabidopsis. Plant Cell [Internet] 2013; 25:1416–1429. Available from] ;:. : http://www.plantcell.org/lookup/doi/10.1105/tpc.113.110684. - DOI - PMC - PubMed
-
- Behnke J, Feige MJ, Hendershot LM.. BiP and its nucleotide exchange factors Grp170 and Sil1: mechanisms of action and biological functions HHS public access. J Mol Biol [Internet] 2015. 427:1589–1608. [cited 2019 June12]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4356644/pdf/nihms664816.pdf. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
