Burden of respiratory syncytial virus hospitalisation among infants born at 32-35 weeks' gestational age in the Northern Hemisphere: pooled analysis of seven studies
- PMID: 32799945
- PMCID: PMC7439292
- DOI: 10.1017/S0950268820001661
Burden of respiratory syncytial virus hospitalisation among infants born at 32-35 weeks' gestational age in the Northern Hemisphere: pooled analysis of seven studies
Abstract
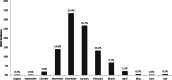
To provide comprehensive information on the epidemiology and burden of respiratory syncytial virus hospitalisation (RSVH) in preterm infants, a pooled analysis was undertaken of seven multicentre, prospective, observational studies from across the Northern Hemisphere (2000-2014). Data from all 320-356 weeks' gestational age (wGA) infants without comorbidity were analysed. RSVH occurred in 534/14 504 (3.7%) infants; equating to a rate of 5.65 per 100 patient-seasons, with the rate in individual wGA groups dependent upon exposure time (P = 0.032). Most RSVHs (60.1%) occurred in December-January. Median age at RSVH was 88 days (interquartile range (IQR): 54-159). Respiratory support was required by 82.0% of infants: oxygen in 70.4% (median 4 (IQR: 2-6) days); non-invasive ventilation in 19.3% (median 3 (IQR: 2-5) days); and mechanical ventilation in 10.2% (median 5 (IQR: 3-7) days). Intensive care unit admission was required by 17.9% of infants (median 6 days (IQR: 2-8) days). Median overall hospital length of stay (LOS) was 5 (IQR: 3-8) days. Hospital resource use was similar across wGA groups except for overall LOS, which was shortest in those born 35 wGA (median 3 vs. 4-6 days for 32-34 wGA; P < 0.001). Strategies to reduce the burden of RSVH in otherwise healthy 32-35 wGA infants are indicated.
Keywords: Epidemiology; Lower respiratory tract infection; Moderate-to-late preterm infants; RSV hospitalisation; Respiratory support; Respiratory syncytial virus.
Conflict of interest statement
EJA, ML, XC-E, MB, MS-P, BP have received research funding and/or compensation as advisor/lecturer from AbbVie. EJA has received research funding from MedImmune, Regeneron, Pfizer, Merck, Novavax, GSK, Sanofi Pasteur, PaxVax and Micron. EJA has received compensation from Pfizer for consulting. BRG and JF, working for Strategen, have previously received payment from AbbVie for work on various projects. EG is an employee of AbbVie and owns stock.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical