Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial
- PMID: 32800099
- PMCID: PMC7491203
- DOI: 10.1016/S1470-2045(20)30398-3
Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial
Abstract
Background: The appropriate age range for breast cancer screening remains a matter of debate. We aimed to estimate the effect of mammographic screening at ages 40-48 years on breast cancer mortality.
Methods: We did a randomised, controlled trial involving 23 breast screening units across Great Britain. We randomly assigned women aged 39-41 years, using individual randomisation, stratified by general practice, in a 1:2 ratio, to yearly mammographic screening from the year of inclusion in the trial up to and including the calendar year that they reached age 48 years (intervention group), or to standard care of no screening until the invitation to their first National Health Service Breast Screening Programme (NHSBSP) screen at approximately age 50 years (control group). Women in the intervention group were recruited by postal invitation. Women in the control group were unaware of the study. The primary endpoint was mortality from breast cancers (with breast cancer coded as the underlying cause of death) diagnosed during the intervention period, before the participant's first NHSBSP screen. To study the timing of the mortality effect, we analysed the results in different follow-up periods. Women were included in the primary comparison regardless of compliance with randomisation status (intention-to-treat analysis). This Article reports on long-term follow-up analysis. The trial is registered with the ISRCTN registry, ISRCTN24647151.
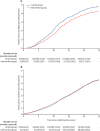
Findings: 160 921 women were recruited between Oct 14, 1990, and Sept 24, 1997. 53 883 women (33·5%) were randomly assigned to the intervention group and 106 953 (66·5%) to the control group. Between randomisation and Feb 28, 2017, women were followed up for a median of 22·8 years (IQR 21·8-24·0). We observed a significant reduction in breast cancer mortality at 10 years of follow-up, with 83 breast cancer deaths in the intervention group versus 219 in the control group (relative rate [RR] 0·75 [95% CI 0·58-0·97]; p=0·029). No significant reduction was observed thereafter, with 126 deaths versus 255 deaths occurring after more than 10 years of follow-up (RR 0·98 [0·79-1·22]; p=0·86).
Interpretation: Yearly mammography before age 50 years, commencing at age 40 or 41 years, was associated with a relative reduction in breast cancer mortality, which was attenuated after 10 years, although the absolute reduction remained constant. Reducing the lower age limit for screening from 50 to 40 years could potentially reduce breast cancer mortality.
Funding: National Institute for Health Research Health Technology Assessment programme.
Copyright © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures

Comment in
-
Final results of the UK Age trial on breast cancer screening age.Lancet Oncol. 2020 Sep;21(9):1125-1126. doi: 10.1016/S1470-2045(20)30428-9. Epub 2020 Aug 12. Lancet Oncol. 2020. PMID: 32800098 No abstract available.
-
Mammography screening for breast cancer-the UK Age trial.Lancet Oncol. 2020 Nov;21(11):e504. doi: 10.1016/S1470-2045(20)30528-3. Lancet Oncol. 2020. PMID: 33152296 No abstract available.
-
Mammography screening for breast cancer-the UK Age trial.Lancet Oncol. 2020 Nov;21(11):e505. doi: 10.1016/S1470-2045(20)30492-7. Lancet Oncol. 2020. PMID: 33152297 No abstract available.
-
Mammography screening for breast cancer-the UK Age trial.Lancet Oncol. 2020 Nov;21(11):e506. doi: 10.1016/S1470-2045(20)30550-7. Lancet Oncol. 2020. PMID: 33152298 No abstract available.
-
Mammography screening for breast cancer-the UK Age trial.Lancet Oncol. 2020 Nov;21(11):e507. doi: 10.1016/S1470-2045(20)30491-5. Lancet Oncol. 2020. PMID: 33152299 No abstract available.
-
Mammography screening for breast cancer-the UK Age trial.Lancet Oncol. 2020 Nov;21(11):e508. doi: 10.1016/S1470-2045(20)30490-3. Lancet Oncol. 2020. PMID: 33152300 No abstract available.
-
Mammography screening for breast cancer-the UK Age trial.Lancet Oncol. 2020 Nov;21(11):e509. doi: 10.1016/S1470-2045(20)30631-8. Lancet Oncol. 2020. PMID: 33152301 No abstract available.
-
Mammography screening for breast cancer-the UK Age trial - Authors' reply.Lancet Oncol. 2020 Nov;21(11):e510. doi: 10.1016/S1470-2045(20)30627-6. Lancet Oncol. 2020. PMID: 33152302 No abstract available.
-
In women aged 40 to 48 y, annual mammography vs. usual care reduced breast cancer mortality at 10 but not 23 y.Ann Intern Med. 2021 Feb;174(2):JC18. doi: 10.7326/ACPJ202102160-018. Epub 2021 Feb 2. Ann Intern Med. 2021. PMID: 33524283
References
-
- Paci E, Broeders M, Hofvind S, Puliti D, Duffy SW. European breast cancer service screening outcomes: a first balance sheet of the benefits and harms. Cancer Epidemiol Biomarkers Prev. 2014;23:1159–1163. - PubMed
-
- IARC Working Group on the Evaluation of Cancer-Preventive Strategies . International Agency for Research on Cancer; Lyon: 2016. Breast cancer screening.
-
- Siu AL. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279–296. - PubMed
-
- Schünemann HJ, Lerda D, Quinn C. Breast cancer screening and diagnosis: a synopsis of the European Breast Guidelines. Ann Intern Med. 2020;172:46–56. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

