The Distributed Nociceptive System: A Framework for Understanding Pain
- PMID: 32800534
- PMCID: PMC7530033
- DOI: 10.1016/j.tins.2020.07.004
The Distributed Nociceptive System: A Framework for Understanding Pain
Abstract
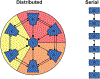
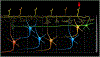
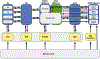
Chronic pain remains challenging to both diagnose and treat. These challenges, in part, arise from limited systems-level understanding of the basic mechanisms that process nociceptive information and ultimately instantiate a subjectively available experience of pain. Here, I provide a framework, the distributed nociceptive system, for understanding nociceptive mechanisms at a systems level by integrating the concepts of neural population coding with distributed processing. Within this framework, wide-spread engagement of populations of neurons produces representations of nociceptive information that are highly resilient to disruption. The distributed nociceptive system provides a foundation for understanding complex spatial aspects of chronic pain and provides an impetus for nonpharmacological cognitive and physical therapies that can effectively target the highly distributed system that gives rise to an experience of pain.
Keywords: bilateral; biomarkers; nociception; pain; population coding; recruitment.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures


References
-
- Neville A et al. (2019) Diagnostic Uncertainty in Youth With Chronic Pain and Their Parents. Journal of Pain 20 (9), 1080–1090. - PubMed
-
- Price DD et al. (1978) Spatial and temporal transformations of input to spinothalamic tract neurons and their relation to somatic sensations. J Neurophysiol 41 (4), 933–47. - PubMed
-
- Sherrington CS (1908) The integrative action of the nervous system, Archibald Constable.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

