A Comparison of Sonothrombolysis in Aged Clots between Low-Boiling-Point Phase-Change Nanodroplets and Microbubbles of the Same Composition
- PMID: 32800631
- PMCID: PMC8146824
- DOI: 10.1016/j.ultrasmedbio.2020.07.008
A Comparison of Sonothrombolysis in Aged Clots between Low-Boiling-Point Phase-Change Nanodroplets and Microbubbles of the Same Composition
Abstract
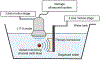
We present enhanced cavitation erosion of blood clots exposed to low-boiling-point (-2°C) perfluorocarbon phase-change nanodroplets and pulsed ultrasound, as well as microbubbles with the same formulation under the same conditions. Given prior success with microbubbles as a sonothrombolysis agent, we considered that perfluorocarbon phase-change nanodroplets could enhance clot disruption further beyond that achieved with microbubbles. It has been hypothesized that owing to their small size and ability to penetrate into a clot, nanodroplets could enhance cavitation inside a blood clot and increase sonothrombolysis efficacy. The thrombolytic effects of lipid-shell-decafluorobutane nanodroplets were evaluated and compared with those of microbubbles with the same formulation, in an aged bovine blood clot flow model. Seven different pulsing schemes, with an acoustic intensity (ISPTA) range of 0.021-34.8 W/cm2 were applied in three different therapy scenarios: ultrasound only, ultrasound with microbubbles and ultrasound with nanodroplets (n = 5). Data indicated that pulsing schemes with 0.35 W/cm2 and 5.22 W/cm2 produced a significant difference (p < 0.05) in nanodroplet sonothrombolysis performance compared with compositionally identical microbubbles. With these excitation conditions, nanodroplet-mediated treatment achieved a 140% average thrombolysis rate over the microbubble-mediated case. We observed distinctive internal erosion in the middle of bovine clot samples from nanodroplet-mediated ultrasound, whereas the microbubble-mediated case generated surface erosion. This erosion pattern was supported by ultrasound imaging during sonothrombolysis, which revealed that nanodroplets generated cavitation clouds throughout a clot, whereas microbubble cavitation formed larger cavitation clouds only outside a clot sample.
Keywords: Cavitation; Clot; Microbubbles; Nanodroplets; Sonothrombolysis.
Copyright © 2020. Published by Elsevier Inc.
Conflict of interest statement
Conflict of interest disclosure P.A.D. is an inventor on patents related to low-boiling-point phase-change nanodroplets, and a co-founder of Triangle Biotechnology, which has licensed some of these patents. J.K. and X.J. are inventors of an intravascular sonothrombolysis patent, which was licensed by SonoVascular, Inc. X.J. is a co-founder of SonoVascular, Inc. P.A.D., X.J., J.K., and Z.X. are inventors on a pending patent describing nanodroplet enhanced sononthrombolysis.
Figures






Similar articles
-
An Analysis of Sonothrombolysis and Cavitation for Retracted and Unretracted Clots Using Microbubbles Versus Low-Boiling-Point Nanodroplets.IEEE Trans Ultrason Ferroelectr Freq Control. 2022 Feb;69(2):711-719. doi: 10.1109/TUFFC.2021.3137125. Epub 2022 Jan 27. IEEE Trans Ultrason Ferroelectr Freq Control. 2022. PMID: 34932475 Free PMC article.
-
In-vitro sonothrombolysis using thick-shelled polymer microbubbles - a comparison with thin-shelled microbubbles.Cardiovasc Ultrasound. 2020 May 4;18(1):12. doi: 10.1186/s12947-020-00194-2. Cardiovasc Ultrasound. 2020. PMID: 32366318 Free PMC article.
-
Combining radiation force with cavitation for enhanced sonothrombolysis.IEEE Trans Ultrason Ferroelectr Freq Control. 2013 Jan;60(1):97-104. doi: 10.1109/TUFFC.2013.2541. IEEE Trans Ultrason Ferroelectr Freq Control. 2013. PMID: 23287916
-
Current Status of Sub-micron Cavitation-Enhancing Agents for Sonothrombolysis.Ultrasound Med Biol. 2023 May;49(5):1049-1057. doi: 10.1016/j.ultrasmedbio.2023.01.018. Epub 2023 Mar 1. Ultrasound Med Biol. 2023. PMID: 36868959 Review.
-
Advances in Sonothrombolysis Techniques Using Piezoelectric Transducers.Sensors (Basel). 2020 Feb 27;20(5):1288. doi: 10.3390/s20051288. Sensors (Basel). 2020. PMID: 32120902 Free PMC article. Review.
Cited by
-
Acoustics at the nanoscale (nanoacoustics): A comprehensive literature review.: Part II: Nanoacoustics for biomedical imaging and therapy.Sens Actuators A Phys. 2021 Dec 1;332(Pt 2):112925. doi: 10.1016/j.sna.2021.112925. Epub 2021 Jun 17. Sens Actuators A Phys. 2021. PMID: 34937992 Free PMC article.
-
Nanodroplet-mediated catheter-directed sonothrombolysis of retracted blood clots.Microsyst Nanoeng. 2021 Jan 6;7:3. doi: 10.1038/s41378-020-00228-9. eCollection 2021. Microsyst Nanoeng. 2021. PMID: 33456783 Free PMC article.
-
Synchronous Intravital Imaging and Cavitation Monitoring of Antivascular Focused Ultrasound in Tumor Microvasculature Using Monodisperse Low Boiling Point Nanodroplets.ACS Nano. 2024 Jan 9;18(1):410-427. doi: 10.1021/acsnano.3c07711. Epub 2023 Dec 26. ACS Nano. 2024. PMID: 38147452 Free PMC article.
-
Rapid synergistic thrombolysis of ischemic stroke guided by high-resolution and high-speed photoacoustic cerebrovascular imaging.Photoacoustics. 2025 Apr 4;43:100722. doi: 10.1016/j.pacs.2025.100722. eCollection 2025 Jun. Photoacoustics. 2025. PMID: 40271379 Free PMC article.
-
Magneto-sonothrombolysis with combination of magnetic microbubbles and nanodroplets.Ultrasonics. 2021 Sep;116:106487. doi: 10.1016/j.ultras.2021.106487. Epub 2021 Jun 6. Ultrasonics. 2021. PMID: 34119875 Free PMC article.
References
-
- Acconcia C, Leung BYC, Manjunath A, Goertz DE. Interactions between individual ultrasound stimulated microbubbles and fibrin clot. Ultrasound Med Biol 2014;40:2134–2150. - PubMed
-
- Acconcia C, Leung BYC, Manjunath A, Goertz DE. The effect of short duration ultrasound pulses on the interaction between individual microbubbles and fibrin clots. Ultrasound Med Biol 2015;41:2774–2782. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

