Comparison of automated SARS-CoV-2 antigen test for COVID-19 infection with quantitative RT-PCR using 313 nasopharyngeal swabs, including from seven serially followed patients
- PMID: 32800855
- PMCID: PMC7422837
- DOI: 10.1016/j.ijid.2020.08.029
Comparison of automated SARS-CoV-2 antigen test for COVID-19 infection with quantitative RT-PCR using 313 nasopharyngeal swabs, including from seven serially followed patients
Abstract
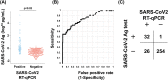
In routine clinical practice, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is determined by reverse-transcription PCR (RT-PCR). In the current pandemic, a more rapid and high-throughput method is in growing demand. Here, we validated the performance of a new antigen test (LUMIPULSE) based on chemiluminescence enzyme immunoassay. A total of 313 nasopharyngeal swabs (82 serial samples from 7 infected patients and 231 individual samples from 4 infected patients and 215 uninfected individuals) were analyzed for SARS-CoV-2 with quantitative RT-PCR (RT-qPCR) and then subjected to LUMIPULSE. We determined the cutoff value for antigen detection using receiver operating characteristic curve analysis and compared the performance of the antigen test with that of RT-qPCR. We also compared the viral loads and antigen levels in serial samples from seven infected patients. Using RT-qPCR as the reference, the antigen test exhibited 55.2% sensitivity and 99.6% specificity, with a 91.4% overall agreement rate (286/313). In specimens with > 100 viral copies and between 10 and 100 copies, the antigen test showed 100% and 85% concordance with RT-qPCR, respectively. This concordance declined with lower viral loads. In the serially followed patients, the antigen levels showed a steady decline, along with viral clearance. This gradual decline was in contrast with the abrupt positive-to-negative and negative-to-positive status changes observed with RT-qPCR, particularly in the late phase of infection. In summary, the LUMIPULSE antigen test can rapidly identify SARS-CoV-2-infected individuals with moderate to high viral loads and may be helpful for monitoring viral clearance in hospitalized patients.
Keywords: Antigen; COVID-19; Immunoassay; Infection; RT-qPCR; SARS-CoV-2.
Copyright © 2020 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- Clerc O., Greub G. Routine use of point-of-care tests: usefulness and application in clinical microbiology. Clin Microbiol Infect. 2010;16:1054–1061. - PubMed
-
- He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X., Lau E.H.Y.C., Wong J.Y., Guan Y., Tan X. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. - PubMed
-
- Hirotsu Y., Maejima M., Nakajima M., Mochizuki H., Omata M. Environmental cleaning is effective for the eradication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in contaminated hospital rooms: a patient from the diamond princess cruise ship. Infect Control Hosp Epidemiol. 2020 doi: 10.1017/ice.2020.144. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

