Engineered PLGA microparticles for long-term, pulsatile release of STING agonist for cancer immunotherapy
- PMID: 32801144
- PMCID: PMC9019818
- DOI: 10.1126/scitranslmed.aaz6606
Engineered PLGA microparticles for long-term, pulsatile release of STING agonist for cancer immunotherapy
Abstract
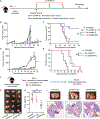
Activation of the stimulator of interferon gene (STING) pathway within the tumor microenvironment has been shown to generate a strong antitumor response. Although local administration of STING agonists has promise for cancer immunotherapy, the dosing regimen needed to achieve efficacy requires frequent intratumoral injections over months. Frequent dosing for cancer treatment is associated with poor patient adherence, with as high as 48% of patients failing to comply. Multiple intratumoral injections also disrupt the tumor microenvironment and vascular networks and therefore increase the risk of metastasis. Here, we developed microfabricated polylactic-co-glycolic acid (PLGA) particles that remain at the site of injection and release encapsulated STING agonist as a programmable sequence of pulses at predetermined time points that mimic multiple injections over days to weeks. A single intratumoral injection of STING agonist-loaded microparticles triggered potent local and systemic antitumor immune responses, inhibited tumor growth, and prolonged survival as effectively as multiple soluble doses, but with reduced metastasis in several mouse tumor models. STING agonist-loaded microparticles improved the response to immune checkpoint blockade therapy and substantially decreased the tumor recurrence rate from 100 to 25% in mouse models of melanoma when administered during surgical resection. In addition, we demonstrated the therapeutic efficacy of STING microparticles on an orthotopic pancreatic cancer model in mice that does not allow multiple intratumoral injections. These findings could directly benefit current STING agonist therapy by decreasing the number of injections, reducing risk of metastasis, and expanding its applicability to hard-to-reach cancers.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures
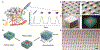
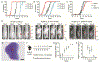



Similar articles
-
Overcome the challenge for intratumoral injection of STING agonist for pancreatic cancer by systemic administration.J Hematol Oncol. 2024 Aug 7;17(1):62. doi: 10.1186/s13045-024-01576-z. J Hematol Oncol. 2024. PMID: 39113096 Free PMC article.
-
Influence of injection technique, drug formulation and tumor microenvironment on intratumoral immunotherapy delivery and efficacy.J Immunother Cancer. 2021 Feb;9(2):e001800. doi: 10.1136/jitc-2020-001800. J Immunother Cancer. 2021. PMID: 33589526 Free PMC article.
-
STING activation reprograms tumor vasculatures and synergizes with VEGFR2 blockade.J Clin Invest. 2019 Jul 25;129(10):4350-4364. doi: 10.1172/JCI125413. J Clin Invest. 2019. PMID: 31343989 Free PMC article.
-
Chemical Biology Perspectives on STING Agonists as Tumor Immunotherapy.ChemMedChem. 2023 Dec 1;18(23):e202300405. doi: 10.1002/cmdc.202300405. Epub 2023 Oct 20. ChemMedChem. 2023. PMID: 37794702 Review.
-
Molecular Pathways: Targeting the Stimulator of Interferon Genes (STING) in the Immunotherapy of Cancer.Clin Cancer Res. 2015 Nov 1;21(21):4774-9. doi: 10.1158/1078-0432.CCR-15-1362. Epub 2015 Sep 15. Clin Cancer Res. 2015. PMID: 26373573 Free PMC article. Review.
Cited by
-
Polymeric microneedle-mediated sustained release systems: Design strategies and promising applications for drug delivery.Asian J Pharm Sci. 2022 Jan;17(1):70-86. doi: 10.1016/j.ajps.2021.07.002. Epub 2021 Jul 24. Asian J Pharm Sci. 2022. PMID: 35261645 Free PMC article. Review.
-
Liposomal Delivery of MIW815 (ADU-S100) for Potentiated STING Activation.Pharmaceutics. 2023 Feb 14;15(2):638. doi: 10.3390/pharmaceutics15020638. Pharmaceutics. 2023. PMID: 36839960 Free PMC article.
-
Responsive Multivesicular Polymeric Nanovaccines that Codeliver STING Agonists and Neoantigens for Combination Tumor Immunotherapy.Adv Sci (Weinh). 2022 Aug;9(23):e2201895. doi: 10.1002/advs.202201895. Epub 2022 Jun 16. Adv Sci (Weinh). 2022. PMID: 35712773 Free PMC article.
-
A single local delivery of paclitaxel and nucleic acids via an immunoactive polymer eliminates tumors and induces antitumor immunity.Proc Natl Acad Sci U S A. 2022 May 31;119(22):e2122595119. doi: 10.1073/pnas.2122595119. Epub 2022 May 24. Proc Natl Acad Sci U S A. 2022. PMID: 35609195 Free PMC article.
-
Chemical and Biomolecular Strategies for STING Pathway Activation in Cancer Immunotherapy.Chem Rev. 2022 Mar 23;122(6):5977-6039. doi: 10.1021/acs.chemrev.1c00750. Epub 2022 Feb 2. Chem Rev. 2022. PMID: 35107989 Free PMC article. Review.
References
-
- Sharma P, Allison JP, The future of immune checkpoint therapy, Science (80-. ). 348, 56–51 (2014). - PubMed
-
- Gajewski TF, Woo S-R, Zha Y, Spaapen R, Zheng Y, Corrales L, Spranger S, Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment, Curr. Opin. Immunol. 25, 268–276 (2013). - PubMed
-
- Galon J, Pagès F, Marincola FM, Angell HK, Thurin M, Lugli A, Zlobec I, Berger A, Bifulco C, Botti G, Tatangelo F, Britten CM, Kreiter S, Chouchane L, Delrio P, Arndt H, Asslaber M, Maio M, V Masucci G, Mihm M, Vidal-Vanaclocha F, Allison JP, Gnjatic S, Hakansson L, Huber C, Singh-Jasuja H, Ottensmeier C, Zwierzina H, Laghi L, Grizzi F, Ohashi PS, Shaw PA, Clarke BA, Wouters BG, Kawakami Y, Hazama S, Okuno K, Wang E, O’Donnell-Tormey J, Lagorce C, Pawelec G, Nishimura MI, Hawkins R, Lapointe R, Lundqvist A, Khleif SN, Ogino S, Gibbs P, Waring P, Sato N, Torigoe T, Itoh K, Patel PS, Shukla SN, Palmqvist R, Nagtegaal ID, Wang Y, D’Arrigo C, Kopetz S, Sinicrope FA, Trinchieri G, Gajewski TF, Ascierto PA, Fox BA, Cancer classification using the Immunoscore: a worldwide task force, J. Transl. Med. 10, 205 (2012). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

