Novel Selective Inhibition of Lactobacillus iners by Lactobacillus-Derived Bacteriocins
- PMID: 32801180
- PMCID: PMC7531956
- DOI: 10.1128/AEM.01594-20
Novel Selective Inhibition of Lactobacillus iners by Lactobacillus-Derived Bacteriocins
Abstract
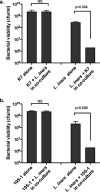
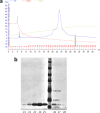
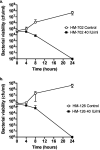
Lactobacillus iners is often associated with vaginal dysbiosis and bacterial vaginosis (BV), which are risk factors for adverse gynecological and obstetric outcomes. To discover natural inhibitors of L. iners, cell-free culture supernatants (CFSs) from 77 vaginal human Lactobacillus strains and 1 human intestinal strain were screened for inhibitory activity. Three active strains were identified, and Lactobacillus paragasseri K7 (K7), a human intestinal strain, produced the most potent L. iners-inhibitory activity. The active material was purified from the K7 CFS and yielded three active peptides, identified as components of two different class IIb, two-peptide bacteriocins, gassericin K7A (GasK7A) and gassericin K7B (GasK7B). The peptides corresponded to the GasK7A α peptide and the GasK7B α and β peptides. While all three peptides exhibited individual activity against L. iners, GasK7B α was the most potent, with an MIC of 23 ng/ml (4 nM). When combined in equal amounts, the GasK7B α and β peptides showed synergistic inhibition, with an MIC of 2 ng/ml (each peptide at 0.4 nM). Among the four major vaginal Lactobacillus species, the K7 bacteriocins selectively inhibited L. iners All 21 strains of L. iners tested (100%) were inhibited by the K7 bacteriocins, whereas <20% of the vaginal Lactobacillus crispatus, L. jensenii, and L. gasseri strains were inhibited. The combination of the BV treatment metronidazole and K7 bacteriocins completely killed both L. iners and Gardnerella vaginalis in a coculture experiment to mimic BV conditions. In contrast, this treatment did not inhibit L. crispatus cultures.IMPORTANCELactobacillus iners is a prevalent species of the vaginal microbiome, but unlike other major vaginal Lactobacillus species, it is not considered protective against BV and can coexist with BV-associated bacteria. L. iners is generally the first Lactobacillus species to emerge following the treatment of BV with metronidazole, and mounting evidence suggests that it may contribute to the onset and maintenance of vaginal dysbiosis. The discovery of highly potent bacteriocins that selectively kill L. iners while sparing protective vaginal lactobacilli may provide novel pharmacological tools to better understand the roles of this enigmatic bacterium in vaginal ecology and potentially lead to new and improved therapies for dysbiosis-related conditions such as BV.
Keywords: Lactobacillus iners; bacterial vaginosis; bacteriocins; vaginal dysbiosis; vaginal microbiome.
Copyright © 2020 American Society for Microbiology.
Figures






Similar articles
-
Practical media formulations for rapid growth of Lactobacillus iners and other vaginal bacteria.Appl Environ Microbiol. 2025 Aug 20;91(8):e0018325. doi: 10.1128/aem.00183-25. Epub 2025 Jul 8. Appl Environ Microbiol. 2025. PMID: 40626727 Free PMC article.
-
Purification and genetic characterization of gassericin E, a novel co-culture inducible bacteriocin from Lactobacillus gasseri EV1461 isolated from the vagina of a healthy woman.BMC Microbiol. 2016 Mar 12;16:37. doi: 10.1186/s12866-016-0663-1. BMC Microbiol. 2016. PMID: 26969428 Free PMC article.
-
Comparison of main lactobacillus species between healthy women and women with bacterial vaginosis.Chin Med J (Engl). 2009 Nov 20;122(22):2748-51. Chin Med J (Engl). 2009. PMID: 19951608
-
Cholesterol-Dependent Cytolysins Produced by Vaginal Bacteria: Certainties and Controversies.Front Cell Infect Microbiol. 2020 Jan 10;9:452. doi: 10.3389/fcimb.2019.00452. eCollection 2019. Front Cell Infect Microbiol. 2020. PMID: 31998661 Free PMC article. Review.
-
Lactobacillus iners: Friend or Foe?Trends Microbiol. 2017 Mar;25(3):182-191. doi: 10.1016/j.tim.2016.11.007. Epub 2016 Dec 1. Trends Microbiol. 2017. PMID: 27914761 Review.
Cited by
-
Assessing the Cervicovaginal Microbiota in the Context of hrHPV Infections: Temporal Dynamics and Therapeutic Strategies.mBio. 2022 Oct 26;13(5):e0161922. doi: 10.1128/mbio.01619-22. Epub 2022 Aug 18. mBio. 2022. PMID: 35980030 Free PMC article. Review.
-
Genomic diversity of genus Limosilactobacillus.Microb Genom. 2022 Jul;8(7):mgen000847. doi: 10.1099/mgen.0.000847. Microb Genom. 2022. PMID: 35838756 Free PMC article.
-
Cervicovaginal microbiota: a promising direction for prevention and treatment in cervical cancer.Infect Agent Cancer. 2024 Apr 19;19(1):13. doi: 10.1186/s13027-024-00573-8. Infect Agent Cancer. 2024. PMID: 38641803 Free PMC article. Review.
-
Menopausal status induces vaginal dysbiosis in women with human papillomavirus infection.Sci Rep. 2024 Mar 26;14(1):7092. doi: 10.1038/s41598-024-56314-9. Sci Rep. 2024. PMID: 38528061 Free PMC article.
-
Metronidazole Treatment Failure and Persistent BV Lead to Increased Frequencies of Activated T- and Dendritic-Cell Subsets.Microorganisms. 2023 Oct 27;11(11):2643. doi: 10.3390/microorganisms11112643. Microorganisms. 2023. PMID: 38004655 Free PMC article.
References
-
- Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SSK, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. 2011. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 108(Suppl 1):4680–4687. doi:10.1073/pnas.1002611107. - DOI - PMC - PubMed
-
- Srinivasan S, Hoffman NG, Morgan MT, Matsen FA, Fiedler TL, Hall RW, Ross FJ, McCoy CO, Bumgarner R, Marrazzo JM, Fredricks DN. 2012. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One 7:e37818. doi:10.1371/journal.pone.0037818. - DOI - PMC - PubMed
-
- Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, Cotch MF, Edelman R, Pastorek JG II, Rao AV, McNellis D, Regan JA, Carey JC, Klebanoff MA, Vaginal Infections and Prematurity Study Group . 1995. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. N Engl J Med 333:1737–1742. doi:10.1056/NEJM199512283332604. - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

