Sharing a β-Glucan Meal: Transcriptomic Eavesdropping on a Bacteroides ovatus-Subdoligranulum variabile-Hungatella hathewayi Consortium
- PMID: 32801182
- PMCID: PMC7531972
- DOI: 10.1128/AEM.01651-20
Sharing a β-Glucan Meal: Transcriptomic Eavesdropping on a Bacteroides ovatus-Subdoligranulum variabile-Hungatella hathewayi Consortium
Abstract
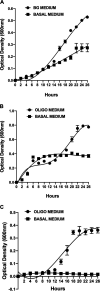
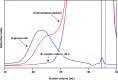
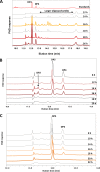
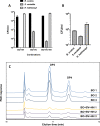
Whole-transcriptome analysis was used to investigate the molecular interplay between three bacterial species that are members of the human gut microbiota. Bacteroides ovatus, Subdoligranulum variabile, and Hungatella hathewayi formed associations in cocultures fed barley β-glucan, a constituent of dietary fiber. B. ovatus depolymerized β-glucan and released, but did not utilize, 3-O-β-cellobiosyl-d-glucose (DP3) and 3-O-β-cellotriosyl-d-glucose (DP4). These oligosaccharides provided growth substrates for S. variabile and H. hathewayi with a preference for DP4 in the case of the latter species. There was increased transcription of a B. ovatus mixed-linkage-β-glucan utilization locus, as well as carbohydrate transporters in S. variabile and H. hathewayi when in batch coculture. Increased transcription of the β-glucan utilization locus did not occur in continuous culture. Evidence for interactions relating to provision of cobalamin, alterations to signaling, and modulation of the "stringent response" (an adaptation to nutrient deprivation) were detected. Overall, we established a bacterial consortium based on barley β-glucan in vitro, which can be used to investigate aspects of the functional blueprint of the human gut microbiota.IMPORTANCE The microbial community, mostly composed of bacterial species, residing in the human gut degrades and ferments polysaccharides derived from plants (dietary fiber) that would not otherwise be digested. In this way, the collective metabolic actions of community members extract additional energy from the human diet. While the variety of bacteria present in the microbial community is well known, the formation of bacterial consortia, and the consequent interactions that result in the digestion of dietary polysaccharides, has not been studied extensively. The importance of our work was the establishment, under laboratory conditions, of a consortium of gut bacteria that formed around a dietary constituent commonly present in cereals. This enabled the metabolic interplay between the bacterial species to be studied. This kind of knowledge is required to construct an interactive, metabolic blueprint of the microbial community that inhabits the human gut.
Keywords: RNAseq; bacterial consortium; beta-glucan; gut microbiota; whole-transcriptome analysis.
Copyright © 2020 American Society for Microbiology.
Figures
References
-
- Plichta DR, Juncker AS, Bertalan M, Rettedal E, Gautier L, Varela E, Manichanh C, Fouqueray C, Levenez F, Nielsen T, Doré J, Machado AMD, de Evgrafov MCR, Hansen T, Jørgensen T, Bork P, Guarner F, Pedersen O, Sommer MOA, Ehrlich SD, Sicheritz-Pontén T, Brunak S, Nielsen HB, Nielsen HB, Metagenomics of the Human Intestinal Tract (MetaHIT) Consortium. 2016. Transcriptional interactions suggest niche segregation among microorganisms in the human gut. Nat Microbiol 1:16152. doi:10.1038/nmicrobiol.2016.152. - DOI - PubMed
-
- Tannock GW. 2017. Understanding the gut microbiota. John Wiley & Sons, Inc, Hoboken, NJ.
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

