Incubation period of COVID-19: a rapid systematic review and meta-analysis of observational research
- PMID: 32801208
- PMCID: PMC7430485
- DOI: 10.1136/bmjopen-2020-039652
Incubation period of COVID-19: a rapid systematic review and meta-analysis of observational research
Abstract
Objectives: The aim of this study was to conduct a rapid systematic review and meta-analysis of estimates of the incubation period of COVID-19.
Design: Rapid systematic review and meta-analysis of observational research.
Setting: International studies on incubation period of COVID-19.
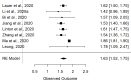
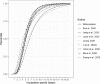
Participants: Searches were carried out in PubMed, Google Scholar, Embase, Cochrane Library as well as the preprint servers MedRxiv and BioRxiv. Studies were selected for meta-analysis if they reported either the parameters and CIs of the distributions fit to the data, or sufficient information to facilitate calculation of those values. After initial eligibility screening, 24 studies were selected for initial review, nine of these were shortlisted for meta-analysis. Final estimates are from meta-analysis of eight studies.
Primary outcome measures: Parameters of a lognormal distribution of incubation periods.
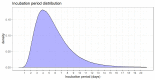
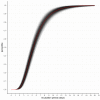
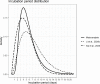
Results: The incubation period distribution may be modelled with a lognormal distribution with pooled mu and sigma parameters (95% CIs) of 1.63 (95% CI 1.51 to 1.75) and 0.50 (95% CI 0.46 to 0.55), respectively. The corresponding mean (95% CIs) was 5.8 (95% CI 5.0 to 6.7) days. It should be noted that uncertainty increases towards the tail of the distribution: the pooled parameter estimates (95% CIs) resulted in a median incubation period of 5.1 (95% CI 4.5 to 5.8) days, whereas the 95th percentile was 11.7 (95% CI 9.7 to 14.2) days.
Conclusions: The choice of which parameter values are adopted will depend on how the information is used, the associated risks and the perceived consequences of decisions to be taken. These recommendations will need to be revisited once further relevant information becomes available. Accordingly, we present an R Shiny app that facilitates updating these estimates as new data become available.
Keywords: epidemiology; public health.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
-
- Brookmeyer R. Incubation period of infectious diseases. Wiley StatsRef: Statistics Reference Online, 2014: 1–8.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
