Clinical-Decision Criteria to Identify Recurrent Diabetic Macular Edema Patients Suitable for Fluocinolone Acetonide Implant Therapy (ILUVIEN®) and Follow-Up Considerations/Recommendations
- PMID: 32801618
- PMCID: PMC7398681
- DOI: 10.2147/OPTH.S252359
Clinical-Decision Criteria to Identify Recurrent Diabetic Macular Edema Patients Suitable for Fluocinolone Acetonide Implant Therapy (ILUVIEN®) and Follow-Up Considerations/Recommendations
Abstract
Current management of diabetic macular edema (DME) predominantly involves treatment with short-acting intravitreal injections of anti-vascular endothelial growth factors (anti-VEGFs) and/or corticosteroids; however, short-acting therapies (lasting between 1 and 6 months) require frequent injections to maintain efficacy, meaning a considerable treatment burden for diabetic patients with multiple comorbidities. Continuous injections needed in some cases are an economic burden for patients/healthcare system, so real-life clinical practice tends to adopt a reactive approach, ie, watch and wait for worsening symptoms, which consequently increases the risk of undertreatment and edema recurrence. On March 7th 2019, a group of experts in retinal medicine and surgery held a roundtable meeting in Madrid, Spain to discuss how to (1) optimize clinical outcomes through earlier use of fluocinolone acetonide (FAc) implant (ILUVIEN®) in patients with persistent or recurrent DME despite therapy; and, (2) to provide guidance to assist physicians in deciding which patients should be treated with ILUVIEN. In this regard, a 36-month follow-up consensus protocol is presented. In conclusion, patients that achieve a complete or partial anatomical, and preferably functional, response following one or two intravitreal dexamethasone implants, but with recurrence of edema after 3-4 months, are deemed by the authors most likely to benefit from ILUVIEN, and the switch to FAc implant should not be delayed more than 12 months after the initiation of at least the first dexamethasone implant.
Keywords: DME; ILUVIEN; diabetic macular edema; fluocinolone acetonide implant therapy.
© 2020 Adán et al.
Conflict of interest statement
Alfredo Adán declares financial support from Allergan, Bayer, Brill Pharma, Alimera and Novartis; Francisco Cabrera and Marta S. Figueroa declare financial support from Alcon, Allergan, Bayer, Brill Pharma, Novartis, and Roche; Patricia Udaondo declares financial support from Alimera, Allergan, Bayer, Bausch and Lomb, Brill Pharma, Graybug, Novartis, Roche; Maximino Abraldes declares financial support from Allergan, Bayer, Brill Pharma, Boehringer Ingelheim, Novartis, Ophthotech and Roche; Miguel Ángel Reyes declares financial support from Alcon, Bayer, Novartis and Brill Pharma; Marta Pazos declares financial support from Allergan, Santen, Brill Pharma, VISUfarma, and Zeiss, including non-financial support from Heidelberg Engineering; and Félix Armadá declares financial support from Bayer, Novartis, Alcon, Medical Mix, Zeiss, Allergan, Bausch & Lomb and Dorc. The authors report no other conflicts of interest in this work.
Figures
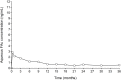
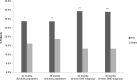

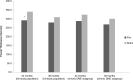


References
-
- Rajendram R, Fraser-Bell S, Kaines A, et al. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. JAMA Ophthalmol. 2012;130(8):972–979. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

