Virucidal Action Against Avian Influenza H5N1 Virus and Immunomodulatory Effects of Nanoformulations Consisting of Mesoporous Silica Nanoparticles Loaded with Natural Prodrugs
- PMID: 32801685
- PMCID: PMC7398888
- DOI: 10.2147/IJN.S247692
Virucidal Action Against Avian Influenza H5N1 Virus and Immunomodulatory Effects of Nanoformulations Consisting of Mesoporous Silica Nanoparticles Loaded with Natural Prodrugs
Abstract
Background: Combating infectious diseases caused by influenza virus is a major challenge due to its resistance to available drugs and vaccines, side effects, and cost of treatment. Nanomedicines are being developed to allow targeted delivery of drugs to attack specific cells or viruses.
Materials and methods: In this study, mesoporous silica nanoparticles (MSNs) functionalized with amino groups and loaded with natural prodrugs of shikimic acid (SH), quercetin (QR) or both were explored as a novel antiviral nanoformulations targeting the highly pathogenic avian influenza H5N1 virus. Also, the immunomodulatory effects were investigated in vitro tests and anti-inflammatory activity was determined in vivo using the acute carrageenan-induced paw edema rat model.
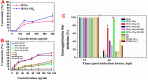
Results: Prodrugs alone or the MSNs displayed weaker antiviral effects as evidenced by virus titers and plaque formation compared to nanoformulations. The MSNs-NH2-SH and MSNs-NH2-SH-QR2 nanoformulations displayed a strong virucidal by inactivating the H5N1 virus. They induced also strong immunomodulatory effects: they inhibited cytokines (TNF-α, IL-1β) and nitric oxide production by approximately 50% for MSNs-NH2-SH-QR2 (containing both SH and QR). Remarkable anti-inflammatory effects were observed during in vivo tests in an acute carrageenan-induced rat model.
Conclusion: Our preliminary findings show the potential of nanotechnology for the application of natural prodrug substances to produce a novel safe, effective, and affordable antiviral drug.
Keywords: immunomodulatory and anti-inflammatory; influenza H5N1 virus; mesoporous silica nanoparticles; nanoformulations-drug delivery system; shikimic acid and quercetin natural prodrugs; virucidal action.
© 2020 AbouAitah et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures





References
-
- Alam MI, Mostafa A, Kanrai P, et al. Verapamil has antiviral activities that target different steps of the influenza virus replication cycle. J Antivir Antiretrovir. 2016;8(4):121–130.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

